
NCERT Solutions For Class 11 Chemistry chapter 12-Some Basic Principles and Techniques
NCERT Solutions for class-11 Chemistry Chapter 12 Some Basics Principles and Techniques is prepared by our senior and renown teachers of Physics Wallah primary focus while solving these questions of class-11 in NCERT textbook, also do read theory of this Chapter 12 Some Basics Principles and Techniques while going before solving the NCERT questions. Our Physics Wallah team Prepared Other Subjects NCERT Solutions for class 11.
Chapter 12 Some Basics Principles and Techniques
Answer the following Questions.
1. Mention the hybridisation states of each carbon atom in the following compounds
CH 2 = C = O, CH 3 CH = CH 2 , (CH 3 ) 2 CO, CH 2 = CHCN, C 6 H 6
Answer:
(i) 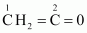
C–1 is sp 2 hybridised.
C–2 is sp hybridised.
(ii) 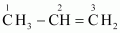
C–1 is sp 3 hybridised.
C–2 is sp 2 hybridised.
C–3 is sp 2 hybridised.
(iii) 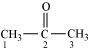
C–1 and C–3 are sp 3 hybridised.
C–2 is sp 2 hybridised.
(iv) 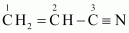
C–1 is sp 2 hybridised.
C–2 is sp 2 hybridised.
C–3 is sp hybridised.
(v) C 6 H 6
All the 6 carbon atoms in benzene are sp2 hybridised.
2. Indicate the σ and π bonds in the following molecules:
C 6 H 6 , C 6 H 12 , CH 2 Cl 2 , CH 2 = C = CH 2 , CH 3 NO 2 , HCO NHCH 3
Answer:
(i) C 6 H 6 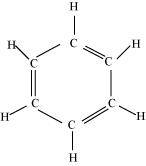
There are six C-C sigma bonds , six C-H bonds ,
and three C=C resonating bonds in the given compound.
(ii) C 6 H 12 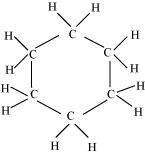
There are six C-C sigma bonds , twelve C-H bonds sigma in the given compound.
(iii) CH 2 C l2, 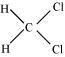
There are two C-H sigma bonds , two C-Cl bonds sigma in the given compound.
(iv)CH 2 = C = CH 2 , 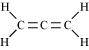
There are two C-C sigma bonds , four C-H bonds and two
C=C π bonds in the given compound.
(v)CH 3 NO 2 , 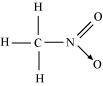
There are three C-H sigma bonds , one C-N sigma bond and one N-O sigma bond and one
N=O π bond in the given compound.
(vi) HCONHCH 3 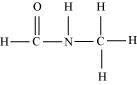
There are two C-N sigma bonds , four C-H sigma bond , N-H sigma bond and one
C=O π bond in the given compound.
3. What are the bond line formulas for the following compounds?
(a)2, 3–dimethyl butanal
(b) Heptan–4–one
(c) Isopropyl alcohol
Answer:
(a)2, 3–dimethyl butanal 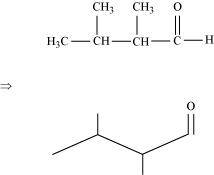
(b) Heptan–4–one 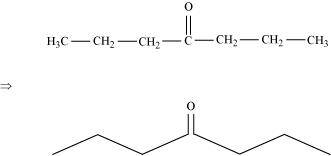
(c) Isopropyl alcohol 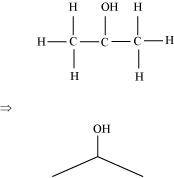
4. Give the IUPAC names of the following compounds:
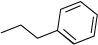
(a)
(b)
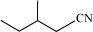
(c)
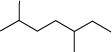
(d) 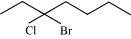
(e)
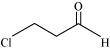
(f) Cl 2 CH CH 2 OH
Answer:
(a)
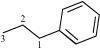
3–phenyl propane
(b)
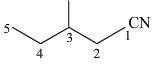
2–methyl–1–cyanobutane
(c)
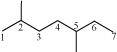
2, 5–dimethyl heptane
(d)
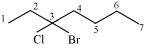
3–bromo–3–chloroheptane
(e)
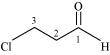
3–chloropropanal
(f) Cl 2 CH CH 2 OH
2,2- Dichloroethanol
5. Which of the following names of the respective compounds are the correct IUPAC-prescribed ones?
(a) 2,2-Dimethyl pentane or 2-Dimethyl pentane
(b) 2,4,7-Trimethyl octane or 2,5,7-Trimethyl octane
(c) 2-Chloro -4-methyl pentane or 4-Chloro-2-methyl pentane
(d) But-3-yn-1-ol or But-4-ol-1-yne
Answer:
(a)The prefix di shows that there are two methyl groups in the chain. Thus the correct IUPAC name would be 2,2-Dimethyl pentane.
(b)The locant number should start from the minimum. Here, 2,4,7 is lower than 2,5,7. Thus, the correct IUPAC name would be 2,4,7-Trimethyloctane.
(c)If the substituents in the chain are in equivalent positions, then the lower number is given to the substituent group in an alphabetical order.Thus, the correct IUPAC name would be 2-Chloro-4-methyl pentane.
(d)Out of the two functional groups present in the given compound, the alcoholic group is the principal functional group. Thus, the parent chain will have an –ol suffix. Since, the alkyne group is in C–3, the IUPAC name would be But–3–yn–1–ol.
6. What are the formulas for the first five members of each of the homologous series beginning with the below-given compounds?
(a) H– COOH
(b) CH 3 COCH 3
(c) H– CH = CH 2
Answer:
The first five members of each homologous series beginning with the given compounds are
(a)
H – COOH :Methanoic acid
CH 3 – COOH :Ethanoic acid
CH 3 – CH 2 – COOH :Propanoic acid
CH 3 – CH 2 – CH 2 – COOH :Butanoic acid
CH 3 – CH 2 – CH 2 – CH 2 – COOH :Pentanoic acid
(b)
CH 3 COCH 3 :Propanone
CH 3 COCH 2 CH 3 : Butanone
CH 3 COCH 2 CH 2 CH 3 : Pentan-2-one
CH 3 COCH 2 CH 2 CH 2 CH 3 : Hexan-2-one
CH 3 COCH 2 CH 2 CH 2 CH 2 CH 3 : Heptan-2-one
(c)
H– CH = CH 2 :Ethene
CH 3 – CH = CH 2 : Propene
CH 3 – CH 2 – CH = CH 2 : 1-Butene
CH 3 – CH 2 – CH 2 – CH = CH 2 : 1-Pentene
CH 3 – CH 2 – CH 2 – CH 2 – CH = CH 2 : 1-Hexene
7. Write thebond line and condensed structural formulas and also find out the functional group for the following compounds.
(a)2,2,4-Trimethyl pentane
(b)2-Hydroxy-1,2,3-propanetri carboxylic acid
(c) Hexanedial
Answer:
(a) 2, 2, 4–trimethyl pentane
Condensed formula
(CH 3 ) 2 CHCH 2 C (CH 3 ) 3
Bond line formula : 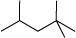
(b) 2–hydroxy–1, 2, 3–propanetri carboxylic acid
Condensed Formula
(COOH) CH 2 C (OH) (COOH) CH 2 (COOH)
Bond line formula:
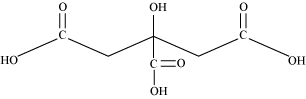
Functional groups:
Carboxylic acid(-COOH) and Alcoholic (-OH) groups
(c) Hexanedial
Condensed Formula
(CHO)(CH 2 ) 4 (CHO)
Bond line formula:
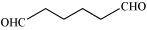
Functional groups:
Aldehyde(-CHO)
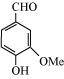
8. What are the functional groups present in the below given compounds?
(a)
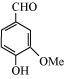
(b)
(c) 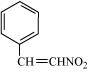
Answer:
(a) Hydroxyl (–OH),Aldehyde (–CHO), Methoxy (–OMe),
C=C double bond 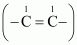
(b)Ketone (C = O),Amino (–NH 2 ),Diethylamine (N(C 2 H 5 ) 2 )
(c) Nitro (–NO 2 ),
C=C double bond 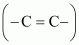
9. Which of the two given compounds: O 2 NCH 2 CH 2 O– or CH 3 CH 2 O– would you expect to be more stable and why?
Answer:
Since NO 2 belongs to the electron-withdrawing group, it shows –I effect. NO 2 tries to decrease the negative charge on the compound by withdrawing the electrons toward it. This stabilizes the compound whereas ethyl group belongs to the electron-releasing group and shows +I effect. This results in an increase in the negative charge on the compound thus destabilizing the compound. Hence, I would expect O 2 NCH 2 CH 2 O – to be more stable than CH 3 CH 2 O –.
10. Why do you think alkyl groups act as electron donors when they get attached to a π system?
Answer:
Due to hyperconjugation, an alkyl group behaves as an electron-donor group when attached to a π system.For example, look at propene.
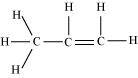
The sigma electrons of the C-H bond get delocalized due to hyperconjugation. The alkyl is attached directly to an unsaturated system. The delocalization happens due to the partial overlap of a sp 3 -s sigma bond orbital with an empty p orbital of the n bond of an adjacent carbon atom.
This process can be shown as: 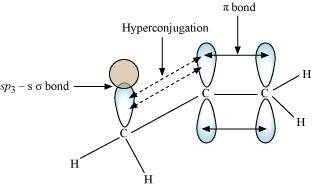
This type of overlap leads to a delocalisation (also known as no-bond resonance) of the π electrons, making the molecule more stable.
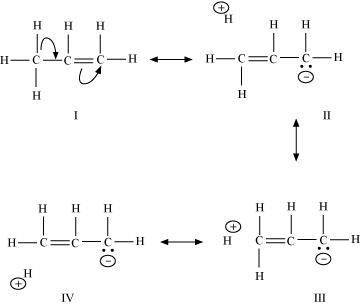
11. Sketch the resonance structures of the below given compounds along with a curved arrow notation to show the electron shift.
(a) C 6 H 5 OH
(b) C 6 H 5 NO 2
(c) CH 3 CH = CH – CHO
(d) C 6 H 5 CHO
(e) C 6 H 5 - CH 2
(f)CH 3 CH = CHCH 2 ⊕
Answer:
(a) The structure of C 6 H 5 OH is: 
Resonating structures: 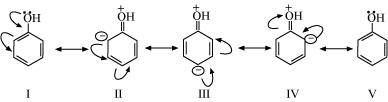
(b) The structure of C 6 H 5 NO 2 is: 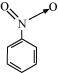
Resonating structures: 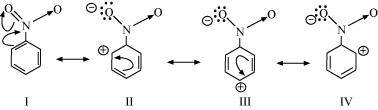
(c) CH 3 CH = CH – CHO
The resonating structures of the given compound are represented as: 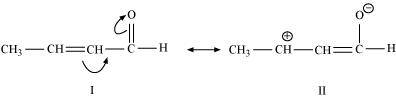
(d) The structure of C 6 H 5 CHO is: 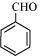
Resonating structures: 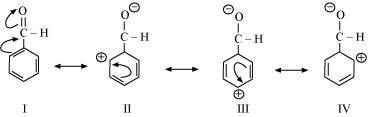
(e) C 6 H 5 - CH 2
Resonating structures: 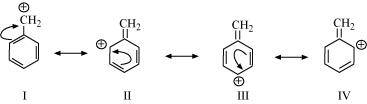
(f) CH 3 CH=CHCH 2 ⊕
Resonating structures: 
12. Define Nucleophiles and Electrophiles with the help of examples.
Answer:
A nucleophile is a reagent that has an electron pair and is willing to donate it. It is also known as a nucleus-loving reagent. Ex: NC – , OH – , R 3 C – (carbanions) etc.
An electrophile is a reagent which is in need of an electron pair and is also known as an electron-loving pair. Ex: Carbonyl groups, CH 3 CH 2 + ( 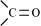 Carbocations), Neutral molecules( due to the presence of a lone pair).
Carbocations), Neutral molecules( due to the presence of a lone pair).
13. Classify the following reagents as Electrophiles or Nucleophiles.
(a) CH 3 COOH + HO – → CH 3 COO – + H 2 O
(b) CH 3 COCH 3 + C–N → (CH3) 2 C(CN) + (OH) –
(c) C 6 H 5 + CH 3 C+ O → C 6 H 5 COCH 3
Answer:
A nucleophile is a reagent that has an electron pair and is willing to donate it. It is also known as a nucleus-loving reagent.
An electrophile is a reagent which is in need of an electron pair and is also known as an electron-loving pair.
(a) CH 3 COOH + HO – → CH 3 COO – + H 2 O
It is a nucleophile since HO- is electron rich in nature.
(b) CH 3 COCH 3 + C–N → (CH 3 ) 2 C(CN) + (OH) –
It is a nucleophile since C–N is electron rich in nature.
(c) C 6 H 5 + CH 3 C + O → C 6 H 5 COCH 3
It is an electrophile since CH 3 C + O is electron-deficient in nature.
14. Find out the type of reaction happening in each of the following equations:
(a) CH 3 CH 2 Br + HS – → CH 3 CH 2 SH + Br –
(b) (CH 3 ) 2 C=CH2 + HCl→ (CH 3 ) 2 ClC – CH 3
(c) CH 3 CH 2 Br + HO – → CH 2 =CH 2 + H2O + Br –
(d) (CH 3 ) 3 C–CH2 OH + HBr → (CH 3 ) 2 CBr CH 2 CH 3 + H 2 O
Answer:
(a) Substitution reaction since bromine group gets substituted by –SH group.
(b) Addition reaction since two reactant molecules combines to form a single product.
(c) Elimination reaction since reaction hydrogen and bromine are removed to form ethene.
(d) Substitution reaction since rearrangement of atoms takes place.
15. Are the below given sets of compounds related to each other? If so, are they geometrical or structural isomers or resonance contributors?
(a)

(b)
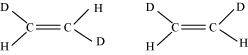
(c) 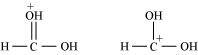
Answer :
(a) Compounds having the same molecular formula but with different structures are called structural isomers. The given compounds have the same molecular formula but they differ in the position of the functional group (ketone group). 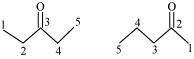
In structure I, ketone group is at the C-3 of the parent chain (hexane chain) and in structure II, ketone group is at the C-2 of the parent chain (hexane chain). Hence, the given pair represents structural isomers.
(b) Compounds having the same molecular formula, the same constitution, and the sequence of covalent bonds, but with different relative position of their atoms in space are called geometrical isomers. 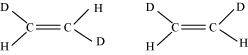
In structures I and II, the relative position of Deuterium (D) and hydrogen (H) in space are different. Hence, the given pairs represent geometrical isomers.
(c) The given structures are canonical structures or contributing structures. They are hypothetical and individually do not represent any real molecule. Hence, the given pair represents resonance structures, called resonance isomers. 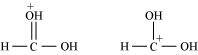
16. For each of the below given bond cleavages, show the electron flow by using curved arrows and classify them as heterolysisor homolysis. Classify the intermediate produced as carbocation or free radical or carbanion.
(a)

(b)

(c)
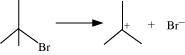
(d) 
Answer:
(a) The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as 
It is an example of homolytic cleavage as one of the shared pair in a covalent bond goes with the bonded atom. The reaction intermediate formed is a free radical.
(b)The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as 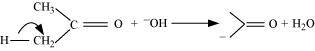
It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the carbon of propanone. The reaction intermediate formed is carbanion.
(c)The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as 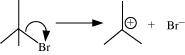
It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the bromine ion. The reaction intermediate formed is a carbocation.
(d)The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as 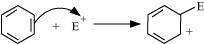
It is a heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with one of the fragments. The intermediate formed is a carbocation.
17. Define the terms Electromeric and Inductive effects. Which effect of electron displacement do you think can explain the below given correct orders of acidity of the carboxylic acids?
(a) Cl 3 C COOH > Cl 2 CH COOH > ClCH 2 COOH
(b) CH 3 CH 2 COOH > (CH 3 ) 2 CH COOH > (CH 3 ) 3 C. COOH
Answer:
Inductive effect
The permanent displacement of sigma (σ) electrons along a saturated chain, whenever an electron withdrawing or electron donating group is present, is called inductive effect. 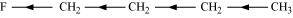
Inductive effect could be + I effect or – I effect. When an atom or group attracts electrons towards itself more strongly than hydrogen, it is said to possess – I effect. For example, 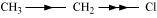
When an atom or group attracts electrons towards itself less strongly than hydrogen, it is said to possess + I effect. For example,
Electrometric effect
It involves the complete transfer of the shared pair of π electrons to either of the two atoms linked by multiple bonds in the presence of an attacking agent. For example, 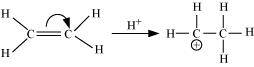
Electrometric effect could be + E effect or – E effect.
+ E effect:When the electrons are transferred towards the attacking reagent
– E effect:When the electrons are transferred away from the attacking reagent
(a)Cl 3 C COOH > Cl 2 CH COOH > ClCH 2 COOH
The order of acidity can be explained on the basis of Inductive effect (– I effect). As the number of chlorine atoms increases, the – I effect increases. With the increase in – I effect, the acid strength also increases accordingly. 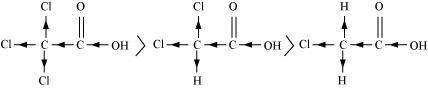
(b)CH 3 CH 2 COOH > (CH 3 ) 2 CHCOOH > (CH 3 ) 3 C.COOH
The order of acidity can be explained on the basis of inductive effect (+ I effect). As the number of alkyl groups increases, the + I effect also increases. With the increase in + I effect, the acid strength also increases accordingly. 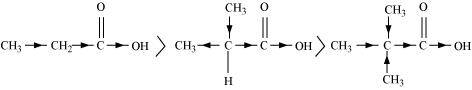
18. Explain the following techniques with the help of examples.
(a)Distillation
(b) Crystallisation
(c) Chromatography
Answer:
(a) Crystallisation
Crystallization is used to purify solid organic compounds.
Principle: The principle on which it works is the difference in the solubility of the compound and impurities in a given solvent. The impure compound is made to dissolve in the solvent at a higher temperature since it is sparingly soluble at lower temperatures. This is continued till we get an almost saturated solution. On cooling and filtering it, we get its’ crystals. Ex: By crystallizing 2-4g of crude aspirin in 20mL of ethyl alcohol, we get pure aspirin. It is heated if needed and left undisturbed until it crystallizes. The crystals are then separated and dried.
(b) Distillation
This method is used to separate non-volatile liquids from volatile impurities. It is also used when the components have a considerable difference in their boiling points.
Principle: The principle on which it works is that liquids having different boiling points vaporise at different temperatures. They are then cooled and the formed liquids are separated.
Ex: A mixture of aniline (b.p = 457 K) and chloroform (b.p = 334 K) is taken in a round bottom flask having a condenser. When they are heated, Chloroform, vaporizes first due to its high volatility and made to pass through a condenser where it cools down. The aniline is left behind in the round bottom flask.
(c) Chromatography
It is widely used for the separation and purification of organic compounds.
Principle: The principle on which it works is that individual components of a mixture move at different paces through the stationary phase under the influence of mobile phase.
Ex: Chromatography can be used to separate a mixture of blue and red ink. This mixture is placed on chromatogram where the component which is less absorbed by the chromatogram moves faster up the paper than the other component which is almost stationary.
19. Describe the method, which can be used to separate two compounds with different solubilities in a solvent S.
Answer: Fractional crystallisation is the method used for separating two compounds with different solubilities in a solvent S. The process of fractional crystallisation is carried out in four steps.
(a) Preparation of the solution: The powdered mixture is taken in a flask and the solvent is added to it slowly and stirred simultaneously. The solvent is added till the solute is just dissolved in the solvent. This saturated solution is then heated.
(b) Filtration of the solution: The hot saturated solution is then filtered through a filter paper in a China dish.
(c) Fractional crystallisation: The solution in the China dish is now allowed to cool. The less soluble compound crystallises first, while the more soluble compound remains in the solution. After separating these crystals from the mother liquor, the latter is concentrated once again. The hot solution is allowed to cool and consequently, the crystals of the more soluble compound are obtained.
(d) Isolation and drying: These crystals are separated from the mother liquor by filtration. Finally, the crystals are dried.
20. Discuss the chemistry of Lassaigne’s test.
Answer:
Lassaigne’s test
This test is employed to detect the presence of nitrogen, sulphur, halogens, and phosphorous in an organic compound. These elements are present in the covalent form in an organic compound. These are converted into the ionic form by fusing the compound with sodium metal. 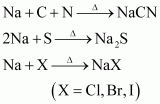
The cyanide, sulphide, and halide of sodium formed are extracted from the fused mass by boiling it in distilled water. The extract so obtained is called Lassaigne’s extract. This Lassaigne’s extract is then tested for the presence of nitrogen, sulphur, halogens, and phosphorous.
(a)Test for nitrogen 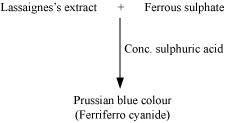
Chemistry of the test
In the Lassaigne’s test for nitrogen in an organic compound, the sodium fusion extract is boiled with iron (II) sulphate and then acidified with sulphuric acid. In the process, sodium cyanide first reacts with iron (II) sulphate and forms sodium hexacyanoferrate (II). Then, on heating with sulphuric acid, some iron (II) gets oxidised to form iron (III) hexacyanoferrate (II), which is Prussian blue in colour. The chemical equations involved in the reaction can be represented as 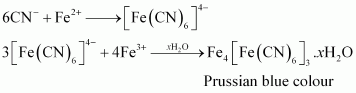
(b)Test for sulphur 
Chemistry of the test
In the Lassaigne’s test for sulphur in an organic compound, the sodium fusion extract is acidified with acetic acid and then lead acetate is added to it. The precipitation of lead sulphide, which is black in colour, indicates the presence of sulphur in the compound. 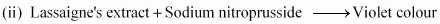
Chemistry of the test
The sodium fusion extract is treated with sodium nitroprusside. Appearance of violet colour also indicates the presence of sulphur in the compound. 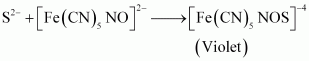
If in an organic compound, both nitrogen and sulphur are present, then instead of NaCN, formation of NaSCN takes place.
Na + C + N + S → NaSCN
This NaSCN (sodium thiocyanate) gives a blood red colour. Prussian colour is not formed due to the absence of free cyanide ions. 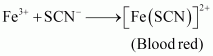
(c)Test for halogens 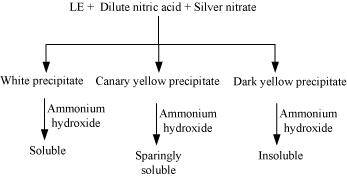
Chemistry of the test
In the Lassaigne’s test for halogens in an organic compound, the sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. 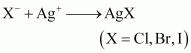
If nitrogen and sulphur both are present in the organic compound, then the Lassaigne’s extract is boiled to expel nitrogen and sulphur, which would otherwise interfere in the test for halogens.
20. What is the difference between distillation, distillation under reduced pressure and steam distillation ?
Answer:
The differences among distillation, distillation under reduced pressure, and steam distillation are given in the following table.
|
Distillation |
Distillation under reduced pressure |
Steam distillation |
|
|
1. |
It is used for the purification of compounds that are associated with non-volatile impurities or those liquids, which do not decompose on boiling. In other words, distillation is used to separate volatile liquids from non-volatile impurities or a mixture of those liquids that have sufficient difference in boiling points. |
This method is used to purify a liquid that tends to decompose on boiling. Under the conditions of reduced pressure, the liquid will boil at a low temperature than its boiling point and will, therefore, not decompose. |
It is used to purify an organic compound, which is steam volatile and immiscible in water. On passing steam, the compound gets heated up and the steam gets condensed to water. After some time, the mixture of water and liquid starts to boil and passes through the condenser. This condensed mixture of water and liquid is then separated by using a separating funnel. |
|
2. |
Mixture of petrol and kerosene is separated by this method. |
Glycerol is purified by this method. It boils with decomposition at a temperature of 593 K. At a reduced pressure, it boils at 453 K without decomposition. |
A mixture of water and aniline is separated by steam distillation. |
NCERT Solutions For Class 11 Chemistry Chapter Wise.









