
NCERT Solutions for class-11 Chemistry Chapter 6: This chapter deals with the study of energy changes, especially heat, during chemical reactions and physical processes. it includes Work, Heat, and Internal Energy (U), Enthalpy (H), Hess’s Law etc.
Class 11 Chemistry Chapter 6 NCERT Solutions
Thermodynamics studies energy changes in chemical and physical processes, explaining heat and work transfer between a system and its surroundings. It introduces internal energy, enthalpy, entropy, and Gibbs free energy, helping predict whether a reaction is spontaneous and understand the energy aspects of chemical changes
NCERT Class 11 Chemistry Chapter 6 Exercise Solutions
NCERT Class 11 Chemistry Chapter 6 Exercise Solutions for Thermodynamics help students understand energy changes, enthalpy, entropy, and Gibbs free energy in chemical reactions.
These NCERT solutions make it easier to solve numerical problems and revise key concepts for exams.
Answer the following Questions.
1. Choose the correct answer. A thermodynamic state function is a quantity
(i) used to determine heat changes
(ii) whose value is independent of path
(iii) used to determine pressure volume work
(iv) whose value depends on temperature only
Solution :
A thermodynamic state function is a quantity Whose value is independent of a path. Functions like p, V, T etc. depend only on the state of a system and not on the path.
Hence, alternative (ii) is correct.
2. For the process to occur under adiabatic conditions, the correct condition is:
(i) ∆T = 0
(ii) ∆p = 0
(iii) q = 0
(iv) w = 0
Solution : A system is said to be under adiabatic conditions if there is no exchange Of heat between the system and its surroundings. Hence, under adiabatic conditions, q = 0.
Therefore, alternative (iii) is correct,
3. The enthalpies of all elements in their standard states are:
(i) unity
(ii) zero
(iii) < 0
(iv) different for each element
Solution : The enthalpy of all elements in their standard state is zero. Therefore, alternative (ii) is correct
4. ΔU ⊖ of combustion of methane is −X kJ mol −1 . The value of ΔH⊖ is
(i)=ΔU ⊖
(ii) >ΔU ⊖
(iii) <=ΔU ⊖
(iv) = 0
Solution :
SinceΔH θ =ΔU θ +Δn g RT and ΔU θ =−Xkmol −1
ΔH θ =(−X)+Δn g RT
⇒△H θ <ΔU θ
Therefore, alternative (iii) is correct.
5. The enthalpy of combustion of methane, graphite and dihydrogen at 298 K are, –890.3kJmol −1 −393.5kJmol −1 , and −285.8kJmol −1
respectively. Enthalpy of formation of CH 4 will be
(i)−74.8kJmol −1
(ii)−52.27kJmol −1
(iii)+74.8kJmol −1
(iv)+52.26kJmol −1
Solution :
According to the question,
(i)CH 4 (g)+2O 2 (g)⟶CO 2 (z)+2H 2 O(g)ΔH=−890.3kJmol −1
(ii)C(x)+O 2 (y)⟶CO 2 (g)ΔH=−393.5kJmol −1
(iii)2H 2 (g)+O 2 (z)⟶2H 2 O(g)
ΔH=−285.8kJmol −1
Thus, the desired equation is the one that represents the formation of CH 4 (g) i.e..,
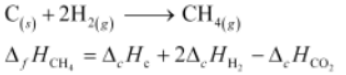
= [-395.5 + 2(-285.8) - (-890.3)] kJ Mol -1
= -74.8 kJ Mol -1
∴ Enthalpy of formation of CH 4 (g)=−74.8kJmol −1 Hence, alternative (i) is correct.
6. A reaction, A + B → C + D + q is found to have a positive entropy change. The reaction will be
(i) possible at high temperature
(ii) possible only at low temperature
(iii) not possible at any temperature
(iv) possible at any temperature
Solution :
For a reaction to be spontaneous, ΔG should be negative.
ΔG = ΔH – TΔS
According to the question, for the given reaction,
ΔS = positive
ΔH = negative (since heat is evolved)
⇒ ΔG = negative
Therefore, the reaction is spontaneous at any temperature.
Hence, alternative (iv) is correct.
7. In a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process?
Solution :
According to the first law of thermodynamics,
ΔU = q + W (i)
Where,
ΔU = change in internal energy for a process
q = heat
W = work
Given,
q = + 701 J (Since heat is absorbed)
W = –394 J (Since work is done by the system)
Substituting the values in expression (i), we get
ΔU = 701 J + (–394 J)
ΔU = 307 J
Hence, the change in internal energy for the given process is 307 J.
8. The reaction of cyanamide,NH 2 CN(s) with dioxygen was carried out in a bomb calorimeter, and ∆U was found to be –742.7kJmol −1 at 298 K. Calculate enthalpy change for the reaction at 298 K.
NH 2 CN(g) + 3/2O 2 (g)→N 2 (g)+CO 2 (g)+H 2 O(l)
Solution :
Enthalpy change for a reaction (ΔH) is given by the expression,
ΔH = ΔU + Δn g RT
Where,
ΔU = change in internal energy
Δn g = change in number of moles
For the given reaction,
Δng = ∑n g (products) – ∑n g (reactants)
= (2 – 1.5) moles
Δn g = 0.5 moles
And,
ΔU = –742.7 kJ mol –1
T = 298 K
R = 8.314 × 10 –3 kJ mol –1 K –1
Substituting the values in the expression of ΔH:
ΔH = (–742.7 kJ mol –1 ) + (0.5 mol) (298 K) (8.314 × 10 –3 kJ mol –1 K –1 )
= –742.7 + 1.2
ΔH = –741.5 kJ mol –1
9. Calculate the number of kJ of heat necessary to raise the temperature of 60.0 g of aluminium from 35°C to 55°C. Molar heat capacity of Al is 24 Jmol −1 K −1 .
Solution :
From the expression of heat (q) q=m⋅ c. ΔT
Where,
c= molar heat capacity
m= mass of substance
ΔT= change in temperature
Substituting the values in the expression of q:
q=(60/27mol)(24Jmol −1 K −1 )(20K)
q=1066.7J
q=1.07k
10. Calculate the enthalpy change on freezing of 1.0 mol of water at10.0°C to ice at
–10.0°C.ΔfusH=6.03kJmol−1 at 0 ∘ C
C p [H 2 O(I)]=75.3Jmol −1 K−1
C ρ [H 2 O(s)]=36.8Jmol −1 K −1
Solution :
Total enthalpy change involved in the transformation is the of the following changes:
(a) Energy change involved in the transformation of 1 mol of water at 10 ∘ C to 1 mol of water at 0 C.
(b) Energy change involved in the transformation of 1 mol of water at 0∘ to 1 mol of ice at 0 ∘ C
(c) Energy change involved in the transformation of 1 mol of ice at0∘C to 1 mol of ice at−10 ∘ C.
ΔH= C p [ H 2 OCl]ΔT+ ΔH fivering + C ρ [H 2 O (s) ]ΔT
=(75.3] mol −1 K −1 )(0−10)K + (−6.03 × 10 3 Jmol −1 )+(36.8] mol −1 K −1 )(−10 −0)K
=−7533 mol −1 − 6030Jmol −1 − 368Jmol −1
=−7151J mol −1
=−7.151kJmol −1
Hence, the enthalpy change involved in the transformation is−7.151kJmol −1 .
11. Enthalpy of combustion of carbon to CO 2 is –393.5−7.151kJmol −1 . Calculate the heat released upon formation of 35.2 g ofCO 2 from carbon and dioxygen gas.
Solution :
Formation of CO 2 from carbon and dioxygen gas can be represented as:
C(s) + O 2 (g)⟶CO 2 (g)
Δ f H=−393.5kJmol −1
(1 mole =44g) Heat released on formation of 44gCO 2 =−393.5kJmol−1
∴ Heat released on formation of 35.2gCO 2
=−314.8kJmol −1
12. Enthalpies of formation of CO(g), CO 2 (g), N 2 O(g) and N 2 O 4 (g) are −110,−393,81 and 9.7kJmol −1 respectively.
Find the value of ∆H for the reaction:
N 2 O 4 (g)+3CO(g)→N 2 O(g+3CO 2 (g]
Solution :
Δ r H for a reaction is defined as the difference between ΔH value of products and ΔH value of reactants.
Δ,H=∑Δ,H( products )−∑Δ f H( reactants )
For the given reaction,
N 2 O 4(g) + 3CO (g) ⟶ N 2 O (g) + 3CO 2 (g)
Δ r H=[ {ΔfH(N 2 O)+3ΔJH(CO 2 )}−{ΔfH (N 2 O 4 ) + 3ΔjH(CO)} ]
Substituting the values ofΔH for N 2 O,CO 2 ,N 2 O 4 , and CO
From the question, we get:
Δ r H=[ { 81kJmol −1 + 3(−393)kJmol −1 } − {9.7kJmol −1 +3(−110) kJmol −1 }]
Δ r H=−7777kJmol −1
Hence, the value ofΔ r H
for the reaction is −777.7 kJmol −1 .
13. Given
N 2 (g) + 3H 2 (g) ⟶ 2NH 3 (y);
Δ r Hθ=−92.4kJmol −1
What is the standard enthalpy of formation ofNH 3 gas?
Solution : Standard of formation of a compound is the charge in enthalpy that takes place during the formation of 1 mole Of a substance in its standard form from its constituent elements in their standard state.
Re-writing the given equation for 1 mole of NH 3(g).
1/2N 2 (g)+3/2H 2 (g)⟶NH 3(g)
∴ Standard enthalpy of formation of NH 3(g)
=1/2 Δ r Hθ = 1/2(−92.4 kJmol −1 )= −46.2kJmol −1
14. Calculate the standard enthalpy of formation ofCH 3 OH(l) from the following data:
CH 3 OH(l)+3/2O 2 (g]→CO 2 (g)+2H 2 O(l): Δ,H∘=−726kJmol −1
C(graphite) +O 2 (g)→CO2(g]: ΔeH=−393kJmol −1
H 2 (g)+1/2O 2 (g)→H 2 O(1); Δ,H=−286kJmol −1
Solution :
The reaction that takes place during the formation ofCH 3 OH(l) can be written as:
C(s) + 2H 2 O(g) + 1/2O 2 (G), ⟶ CH 3 OH( η) (1)
The reaction (I) can be obtained from the given reactions by following the algebraic calculations as:
Equation (ii) + 2 × equation (iii) – equation (i)
Δ f Hθ [CH 3 OH(l)] = ΔcH θ + 2Δ f H θ [H 2 O(l)] – Δ r H θ
= (–393 kJ mol –1 ) + 2(–286 kJ mol –1 ) – (–726 kJ mol –1 )
= (–393 – 572 + 726) kJ mol –1
Δ f H θ [CH 3 OH(l)] = –239 kJ mol –1
15. Calculate the enthalpy change for the process CCl 4 (g)→C(g)+4Cl(g) and calculated bond enthalpy of C−Cl in CCl 4 (g)
Δ va pH θ (CC|4) = 30.5kJmol −1 Δ f H θ (CCl4) =−135.5kJmol−1
Δ a H θ (C) = 715.0kJmol −1 , where Δ a H θ is enthalpy of atomisation
Δ 2 H θ (Cl 2 ) = 242kJmol −1
Solution :
The chemical equations implying to the given values of enthalpies” are:
(1) CCl 4(l) à CCl 4(g) ; ΔvapH Θ = 30.5 kJmol −1
(2) C (s) à C (g) ΔaH Θ = 715 kJmol −1
(3) Cl2 (g) à 2Cl (g) ; Δ a H Θ = 242 kJmol −1
(4) C (g) + 4Cl (g) à CCl 4(g) ; ΔfH Θ
= -135.5 kJmol −1 ΔH for the process CCl 4(g) à C (g) + 4Cl (g) can be measured as:
ΔH=Δ a H Θ (C) + 2ΔaH Θ (Cl 2 ) – Δ vap H Θ –ΔfH
= (715kJmol −1 ) + 2(kJmol −1 ) – (30.5kJmol −1 ) – (-135.5kJmol −1 )
Therefore, H= 1304kJmol −1
The value of bond enthalpy for C-Cl in CCl 4 (g)
= 1304/4kJmol −1
= 326 kJmol −1
16. For an isolated system, ∆U = 0, what will be ∆S ?
Solution :
ΔS will be positive i.e., greater than zero
Since ΔU = 0, ΔS will be positive and the reaction will be spontaneous.
17. For the reaction at 298 K,
2A + B → C
∆H = 400kJmol −1
and ∆S = 0.2kJmol −1
At what temperature will the reaction become spontaneous considering ∆H and ∆S to be constant over the temperature range.
Solution :
From the expression
ΔG= ΔH−TΔS
Assuming the reaction at equilibrium,δ
T for the reaction would be:
T=(ΔH−ΔG)1/ΔS=ΔH/ΔS(ΔG=0 at equilibrium)
=400kJmol −1 0.2 kJK −1 mol −1 T=2000K
For the reaction to be spontaneous,ΔG must be negative. Hence, for the given reaction to be spontaneous, T should be greater than 2000 K.
18. For the reaction,2Cl(g)→Cl 2 (g) , what are the signs of ∆H and ∆S ?
Solution :
∆H and ∆S are negative
The given reaction represents the formation of chlorine molecule from chlorine atoms. Here, bond formation is taking place. Therefore, energy Is being released. Hence ∆H is negative.
Also, two moles of atoms have more randomness than one mole of a molecule. Since spontaneity is decreased, ∆S is negative for the given reaction.
19. For the reaction
2A(g)+B(g)→2D(g)ΔUe=−10.5kJ and ΔS∘=−44.1JK −1
Calculate ΔG ⊖ for the reaction, and predict whether the reaction may occur spontaneously
Solution :
For the given reaction,
2A(g)+B(g)→2D(g)Δηg=2−(3)=−1 mole
Substituting the value of ΔU θ
in the expression of ΔH:
ΔH θ =ΔU θ +Δn g RT
=(−10.5kJ)−(−1)(8.314×10 −3 kJK−1 mol −1 ) (298K) = −10.5kJ −2 .48kJΔH ⊖ =−12.98kJ
Substituting the values ofΔH ⊖ and ΔS ⊖ in the expression of ΔG ⊖
ΔG θ = △H θ −TΔS θ
=−12.98kJ − ( 298K) (−44.1JK −1 ) = −12.98kJ + 13.14 kJ ΔG ⊖ =+0.16kJ
SinceΔG θ for the reaction is positive, the reaction will not occur spontaneously.
20. The equilibrium constant for a reaction is 10. What will be the value of ∆G ⊖ ? R = 8.314JK −1 mol −1
T = 300 K.
Solution :
From the expression, ΔG θ = −2.303 RT logk eq
ΔG θ for the reaction,
=(2.303) (8.314JK −1 mol −1 ) (300K) log10=−5744.14Jmol −1
=−5.744kkmol −1
21. Comment on the thermodynamic stability of NO(g) , given
12N 2(g) +12O 2(g) →NO (g) ;
Δ r H ⊖ = 90kJmol −1 NO (g) + 12O 2(g) →NO 2 (g):
Δ r H e = −74kJmol −1
Solution :
The positive value of Δ r H indicates that heat is absorbed during the formation of NO(g), j. This means that NO(g) has higher than the reactants(N2 and O2) .
Hence, NO (g) is unstable. The negative value o f Δ r H
H indicates that heat is evolved during the formation ofNO 2(g) from NO (g) and O 2(g)
. The product,NO 2(g) is stabilized with minimum energy.
Hence, unstableNO (g) changes to unstableNO 2(g).
22. Calculate the entropy change in surroundings when 1.00 mol ofH 2 O(l) is formed under standard conditions.ΔH θ =−286kJ mol −1
Solution :
It is given that 286 kJmol −1 of heat is evolved the formation of 1 mol ofH 2 O(l).
Thus, an equal amount of heat will be absorbed by the surroundings.
q surr = +286 kJmol −1
Entropy change(ΔS surr ) for the surroundings = q surr / 7
=286kJmol −1 / 298k
∴ΔS surt =959.73 Jmol −1 K −1
Class 11 Chemistry chapter 6 Thermodynamics PDF
Download the NCERT Class 11 Chemistry Chapter 6 – Thermodynamics PDF to access all theory, examples, and exercises for easy study. It helps students revise concepts and solve problems offline.
NCERT Solutions Class 11 Chemistry chapter 6 Thermodynamics
Study without using the internet