Chemical Bonding NEET Mindmaps: Chemical Bonding is an important chapter in Chemistry that lays down the foundation of how atoms interact to make molecules. Topics like Ionic bonds, Covalent bonds, Coordinate bonds, Resonance, Hybridization, and Molecular geometry are covered in the chapter. Most of the students are not able to revise all types of bonds and their exceptions, formulas, and diagrams that are explained in the textbook in a limited time. Hence, it becomes difficult for the aspirants to remember all the information required in the NEET exam.
Chemical Bonding NEET Mindmaps come as a rescue in such situations. Instead of reading the information, all the topics are visually presented in the mindmaps. For example, all the information about ionic bonds and covalent bonds can be connected in one sheet with their types, properties, examples, and rules of stability. The students can make the connections and revise the chapter quickly, which not only saves time but also avoids confusion during revision.
Chemical Bonding NEET Mindmaps
Mindmaps use a central concept or topic from which the sub-topics and other related information are branched out. Chemical Bonding NEET Mindmaps consist of all the important sub-topics that NEET aspirants need to revise, like
-
Ionic bond and Covalent bond
-
Hybridization and its types
-
Molecular shapes
-
Resonance
-
Electronegativity
-
Bond energy and Bond length
The biggest advantage of using mindmaps is that it shows the relationship between different concepts of the chapter. For example, a central node “Covalent Bond” can be connected with branches like polar covalent bonds and non-polar covalent bonds. Each of these sub-topics can be connected to the types of hybridization and molecular geometry, respectively. If needed, graphs and diagrams can be inserted in the mindmaps as well since NEET students are often asked questions that require visual understanding. By using these Chemical Bonding NEET Mindmaps, aspirants are sure that they have revised the chapter well and can remember all important information.
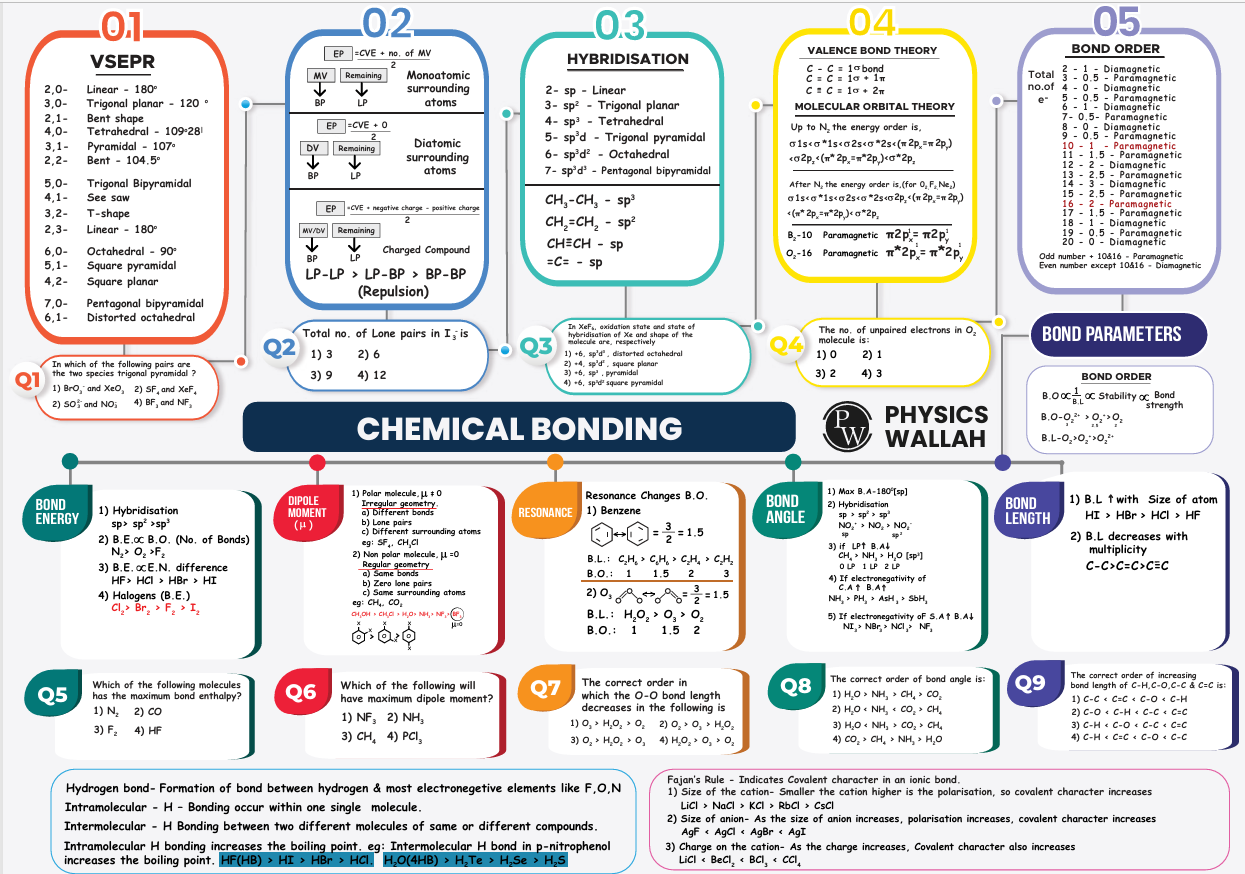
Chemical Bonding NEET Mindmaps PDF
NEET exam preparation requires candidates to maintain accessible study materials. Chemical Bonding NEET mindmap PDF can be carried on mobile phones, tablets, and laptops. The mindmap can be revised at any time even when the students are on the move. They are beneficial for short revision during lunch breaks, while traveling, or during free periods between classes.
The NEET aspirants can also download or print the Chemical Bonding NEET mindmap PDF and paste it on the pages of their notebooks. They can highlight or make notes on their mindmaps to strengthen memory and improve recall. PW provides ready-made NEET mindmap PDF files.The students can use these mindmaps in conjunction with PW NEET notes for detailed explanations and NEET Physics important questions for complete preparation.
Download Chemical Bonding NEET Mindmaps PDF
Chemical Bonding NEET Mindmap Formulas
Formulas are critical in this chapter as many NEET questions involve calculations of bond energy, bond order, and hybridization. The Chemical Bonding NEET mindmap formulas include essential equations and rules needed for solving numerical and conceptual problems. Here’s a table summarizing key Chemical Bonding NEET mindmap formulas:
| Chemical Bonding NEET Mindmap Formulas | ||
| Concept | Formula | Explanation |
| Bond Order | Bond order = (Bonding electrons – Antibonding electrons)/2 | Determines bond stability |
| Lattice Energy | U = k(Q1Q2)/r | Energy released when ions form a lattice |
| Electronegativity Difference | ΔX = | X_A – X_B |
| Dipole Moment | μ = Q × r | Measure of bond polarity |
| Resonance | No formula; represented by resonance structures | Shows delocalized electrons |
Tricks to Lean Chemical Bonding NEET Mindmaps
Learning chemical bonding becomes easier when you use mindmaps effectively. With simple tricks, you can connect concepts, formulas, and examples in a way that helps you remember them faster and revise efficiently before exams.
-
Connect different types of bonds with their properties using arrows and flowcharts for a better understanding of their characteristics.
-
Use different colors to distinguish between ionic bonds, covalent bonds, and coordinate bonds for quick recall.
-
Go through the mindmaps for a few minutes every day to make sure that all the formulas, rules, and exceptions are fresh in your memory.
-
Try to link the nodes of the mindmap with real-life examples like salt formation for ionic bonds and water molecules for polar covalent bonds.
-
Always use your mindmaps along with PW NEET notes and NEET Physics important questions for a complete preparation strategy.
-
These tricks will help the students make the most of the mindmaps and improve their speed and accuracy in solving NEET questions.
Benefits of Chemical Bonding NEET Mindmaps
Mindmaps offer more than just easy visualization. They make revision faster, reduce errors, and help students link different topics, improving overall understanding and confidence during exams.
-
Mindmaps enable the students to revise all the concepts, formulas, and diagrams on a single sheet. It also saves a lot of time that students generally spend in finding a specific formula or rule in their notes.
-
The use of mindmaps also connects all the small topics, like types of bonds and hybridization, molecular shapes and VSEPR theory. This also enhances the understanding of how different topics are related to each other in the chapter.
-
Students can also significantly reduce the mistakes in formula-based and conceptual questions by using mindmaps. This is because they find it easy to recall information from the mindmaps.
-
The mindmaps can also be used in conjunction with other important study materials like PW NEET notes and NEET Physics important questions for complete preparation.
-
The best use of mindmaps is for last-minute revision as it acts like a ready reference for daily or before-exam revision. It also reduces the stress and increases the confidence of the NEET aspirants.
-
By using these benefits, it is clear that Chemical Bonding NEET Mindmaps are one of the most important tools for NEET aspirants who want to score well in the exam.
Prepare for NEET 2026 with Arjuna NEET 3.0, Lakshya NEET 3.0, and Yakeen NEET 3.0 online batches. These courses provide clear lessons and regular practice tests to help you cover the entire syllabus step-by-step.
Chemical Bonding NEET Mindmaps FAQs
Q1: Are mindmaps sufficient for revision of chemical bonding in NEET?
Q2: Do mindmaps include all formulas and exceptions?
Q3: How can the use of mindmaps improve my learning speed?
Q4: Can mindmaps be used along with other NEET materials?