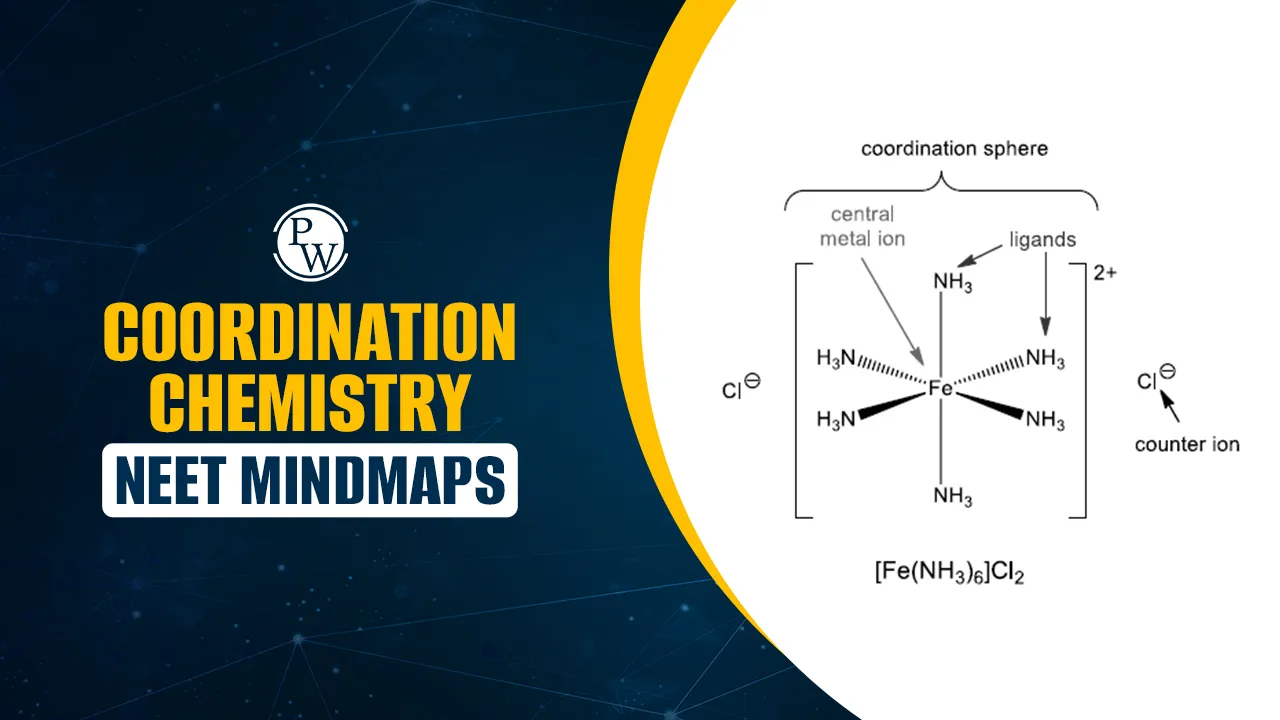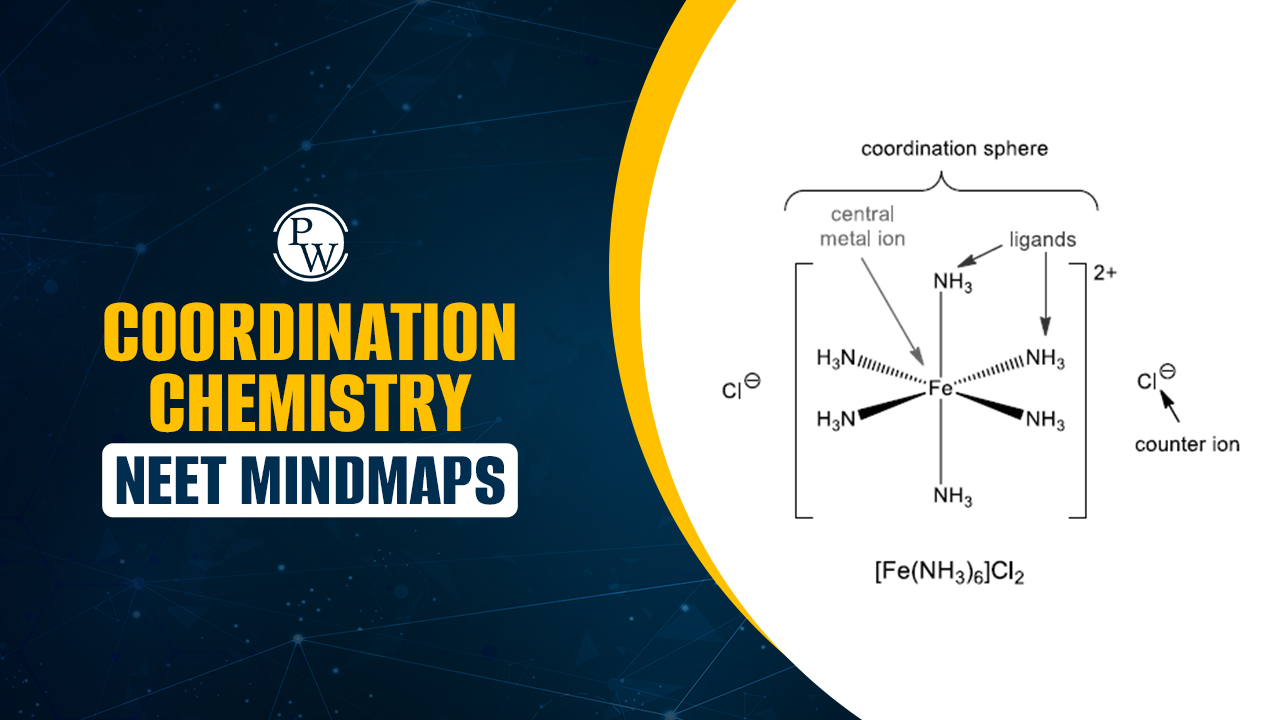
Coordination Chemistry NEET Mindmaps: Coordination chemistry involves the study of coordination compounds formed when central metal ions bond with surrounding ligands through coordinate covalent bonds. It is an important part of the NEET syllabus.
Coordination Chemistry NEET mindmaps help candidates to organize major subtopics, such as classification of ligands, coordination number, and bonding theories. Using the Coordination Chemistry NEET mindmap formulas notes during their preparation for NEET enables efficient revision, logical organization of facts, and boosts problem-solving accuracy.
Coordination Chemistry NEET Mindmaps Overview
Coordination Chemistry is an important part of inorganic chemistry and holds good weightage in NEET exams. It deals with compounds formed when metal ions combine with ligands through coordinate bonds. Coordination Chemistry NEET Mindmaps help students connect ideas easily and remember key points during revision.
Using Coordination Chemistry NEET mindmap notes, students can quickly recall formulas, important rules of IUPAC nomenclature, and trends in stability and reactivity. They also simplify complex topics like hybridization and splitting of d-orbitals.
The main focus areas include coordination number, types of ligands, oxidation states, isomerism, and the naming of coordination compounds. Students should also understand bonding theories like Valence Bond Theory (VBT) and Crystal Field Theory (CFT), which explain the shape, color, and magnetic properties of complexes.
Coordination Chemistry NEET Mindmap PDF
Candidates can download the Coordination Chemistry NEET Mindmap PDF from the link provided below. It is a helpful tool for quick and clear revision for NEET aspirants. Coordination Chemistry NEET Mindmap PDF presents all key concepts like ligands, bonding, and isomerism in one simple chart.
Students can easily revise before exams without going through lengthy notes. It saves time and helps in remembering important formulas and reactions effectively.
Download Coordination Chemistry NEET Mindmap PDF
Coordination Chemistry NEET Mindmap Practice Questions
Here are some Coordination Chemistry NEET Mindmap Practice Questions with explanations and answers to help you revise key concepts clearly:
1. What is the coordination number of the central metal ion in Fe(CN)6]4−[Fe(CN)_6]^{4-}[Fe(CN)6]4−?
Answer: 6
Explanation: The coordination number is equal to the number of ligand donor atoms attached to the central metal. Here, six cyanide (CN⁻) ions are bonded to Fe, so the coordination number is 6.
2. Write the IUPAC name of [Co(NH3)5Cl]Cl2[Co(NH_3)_5Cl]Cl_2[Co(NH3)5Cl]Cl2.
Answer: Pentaamminechloridocobalt(III) chloride
Explanation: The complex contains five ammonia (ammine) ligands and one chloride ligand attached to cobalt. The metal’s oxidation state is +3, found by balancing total charge.
3. What type of isomerism is shown by [Co(NH3)5(NO2)]Cl2[Co(NH_3)_5(NO_2)]Cl_2[Co(NH3)5(NO2)]Cl2?
Answer: Linkage isomerism
Explanation: The NO₂ group can bind through nitrogen (as nitro) or oxygen (as nitrito), leading to linkage isomerism.
4. Which theory explains the magnetic properties of coordination compounds?
Answer: Crystal Field Theory (CFT)
Explanation: CFT explains how the arrangement of ligands affects the d-orbital splitting in the central metal ion, which determines magnetic behavior and color.
5. Differentiate between a chelating ligand and an unidentate ligand with examples.
Answer:
- Chelating ligand: Can attach through two or more donor atoms (e.g., EDTA⁴⁻, oxalate).
- Unidentate ligand: Attaches through only one donor atom (e.g., NH₃, Cl⁻).
Explanation: Chelating ligands form ring-like structures with the metal, increasing stability.
6. Explain why [Cu(NH3)4]2+[Cu(NH_3)_4]^{2+}[Cu(NH3)4]2+ shows a blue color in solution.
Answer: The blue color arises due to d–d electronic transitions within the Cu²⁺ ion. When light is absorbed, an electron jumps between split d-orbitals; the remaining transmitted light appears blue.
Benefits of Using Coordination Chemistry NEET Mindmaps
Coordination Chemistry NEET mindmaps are a useful tool for students to study important topics thoroughly. Mindmaps also improve understanding and help remember concepts better. Here are some benefits outlined:
- Coordination Chemistry NEET mindmap notes help in quick and easy revision before NEET exams.
- The mind maps summarize all important concepts like ligands, geometry, and isomerism together in one place.
- For NEET aspirants, the mindmaps make it easier to remember formulas, reactions, and naming rules.
- Saves time by reducing long notes into short visual points.
- Improves understanding through clear connections between topics.
- Useful for last-minute study and self-checking of weak areas.
- By using the Coordination Chemistry NEET Mindmaps PDF, candidates can link concepts visually and help them retain concepts easily.
- Makes complex topics simple and clear for better NEET preparation.
Join PhysicsWallah's online NEET coaching and make your journey simple and clear. Learn from trusted teachers, follow easy-to-understand lessons, and study at your own pace from home.
Coordination Chemistry NEET Mindmaps FAQs
What is a coordination chemistry mindmap?
How does a mindmap help in NEET preparation?
What topics are covered in coordination chemistry mindmaps?
Where can I get coordination chemistry mindmaps for NEET?
Are coordination chemistry mindmap notes useful for beginners?