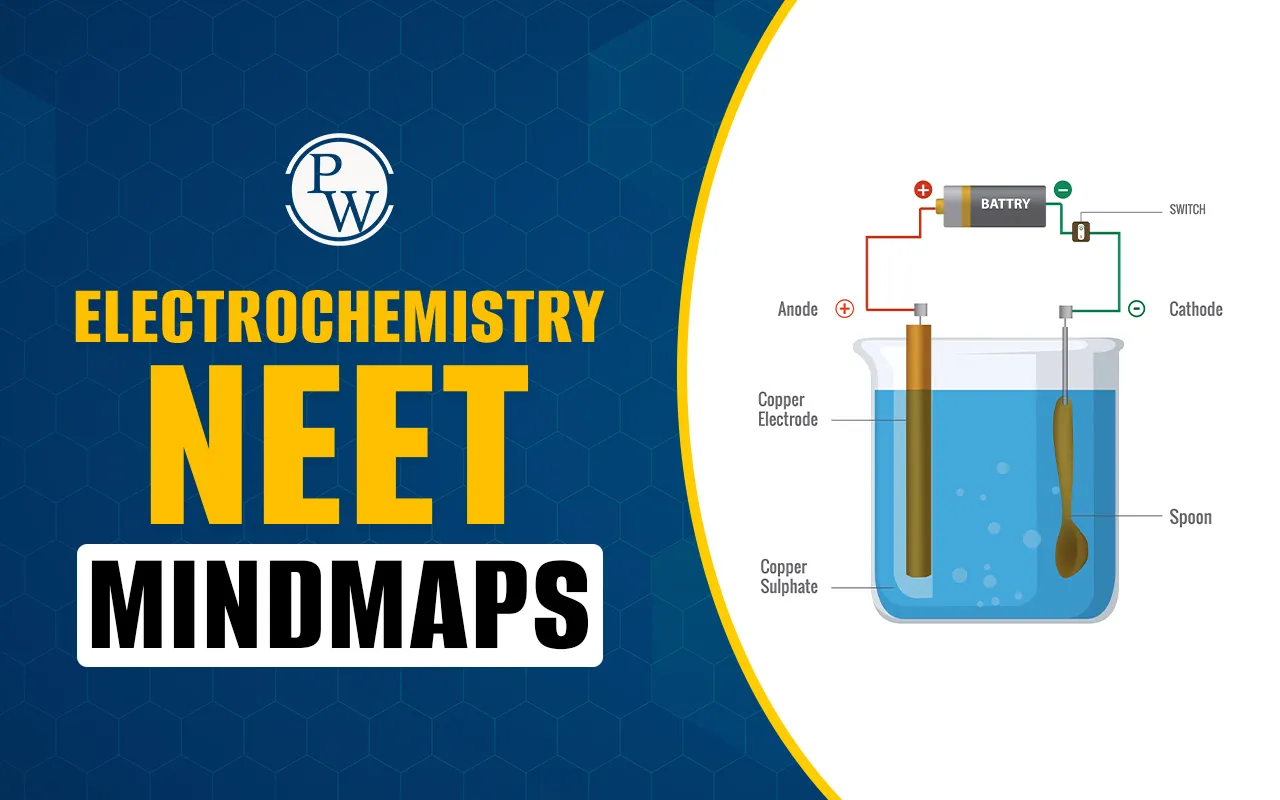
Electrochemistry NEET Mindmaps provide a crucial, condensed view of the entire topic, which is essential for every NEET candidate's final review. This subject deals with the relationship between electrical energy and chemical reactions.
The mindmap organises complex formulas and concepts into easily digestible sections, covering electrochemical cells, electrolytic cells, and important laws. Candidates can solve the Electrochemistry NEET mindmap practice questions.
Electrochemistry NEET Mindmaps
Electrochemistry NEET Mindmaps are considered a practice resource for the candidates. They can learn and revise the important concepts related to Electrochemistry in the paper using the Electrochemistry NEET mindmap notes.
-
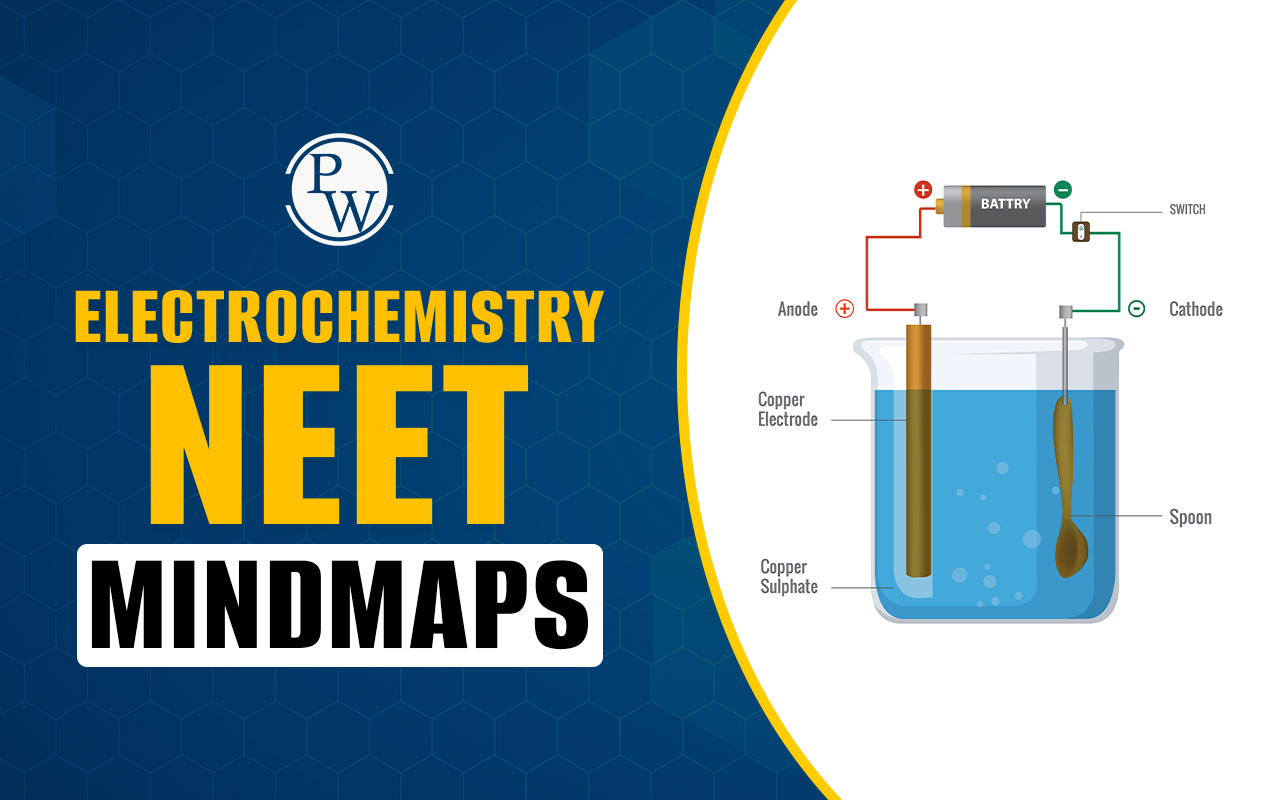
Electrochemical Cell (Galvanic Cell): This cell converts chemical energy into electrical energy. It features oxidation at the anode (negative terminal, left side) and reduction at the cathode (positive terminal, right side).
-
Electrolytic Cell: This cell uses electrical energy to drive non-spontaneous chemical reactions. The anode is positive (where anions go for oxidation), and the cathode is negative (where cations go for reduction).
-
Representation of Cell: A standard notation is used, starting with the anode (oxidation half-cell) on the left, separated by a salt bridge from the cathode (reduction half-cell) on the right.
-
Electrode Potential & EMF: The electromotive force (EMF) of a cell is the potential difference between the two half-cells. It is calculated as Ecell=Ecathode−Eanode.
-
Laws of Electrolysis: Faraday's Laws govern the quantity of product formed during electrolysis.
-
Conductance: Electrolytic conduction is measured using terms like resistance, conductivity, and molar conductivity.
Electrochemistry NEET Mindmap Formulas
Electrochemistry NEET Mindmap Formulas have been compiled in the table. Candidates can revise the formulas and solve the questions. It can help them get the proper understanding of the concepts asked in the paper.
|
Electrochemistry NEET Mindmap Formulas |
||
|
Concept |
Formula |
Description |
|
Nernst Equation (Electrode Potential) |
Ecell = E°cell − (0.0591/n) log([Product]/[Reactant]) (at 298 K) |
Relates the non-standard cell potential (Ecell) to the standard cell potential (E°cell) and concentrations. |
|
EMF and Equilibrium Constant (K) |
log K = (0.0591/n) × E°cell |
Relates the standard cell potential (E°cell) to the equilibrium constant (K). |
|
EMF and Gibbs Free Energy (ΔG) |
ΔG = −nFEcell ; ΔG° = −nFE°cell |
Relates the spontaneity of a reaction (ΔG) to the cell potential (Ecell). |
|
Faraday's Law of Electrolysis |
W = (E × I × t)/96500 OR W = Z × I × t |
Calculates the mass of substance (W) deposited/formed at an electrode. E = equivalent mass, Z = electrochemical equivalent, 96500 C = 1 Faraday. |
|
Molar Conductivity (Λm) |
Λm = (κ × 1000)/M |
Relates conductivity (κ) to molar concentration (M). Unit: S cm² mol⁻¹. |
|
Kohlrausch's Law |
Λm° = xλA° + yλB° (for AxBy) |
The molar conductivity at infinite dilution (Λm°) is the sum of the limiting ionic conductivities of the individual ions. |
Benefits of Solving Electrochemistry NEET Mindmap Practice Questions
Practising questions using these Electrochemistry NEET Mindmaps provides several benefits to the candidate. It can improve the strategy to solve the questions asked in the paper. Therefore, candidates can easily solve the questions with the help of the conceptual understanding.
-
Speed and Accuracy: The visual format helps candidates quickly locate the correct formula or concept needed for a problem.
-
Conceptual Clarity: The mindmap clearly distinguishes between electrochemical and electrolytic processes, preventing confusion on anode/cathode signs.
-
Efficient Revision: Instead of flipping through chapters, candidates can review the entire topic in minutes, focusing on high-yield areas like Nernst equation applications.
-
Predicting Spontaneity: The relationship between ΔG and Ecell allows the candidate to instantly predict if a reaction is spontaneous (if Ecell>0).
Electrochemistry NEET Mindmap PDF
The Electrochemistry NEET mindmap PDF has been provided for the candidates. They can download the file to get the Electrochemistry NEET mindmap notes. A handy resource for the candidates to carry for the last-minute Electrochemistry revision for the NEET examination. Practice and revise for the NEET exam using the mindmaps.
-
Simple language is used for the candidates.
-
Basic to advanced conceptual understanding related to Electrochemistry.
-
Electrochemistry NEET Mindmap is available in PDF format.
-
No internet connection is needed to revise Electrochemistry for the NEET exam.
Electrochemistry NEET Mindmap PDF
Join PhysicsWallah's online NEET coaching and make your journey simple and clear. Learn from trusted teachers, follow easy-to-understand lessons, and study at your own pace from home.
Electrochemistry NEET Mindmaps FAQs
What is the core focus of the Electrochemistry NEET Mindmaps?
In an Electrochemical Cell, which electrode is the anode and what reaction occurs there?
According to the Nernst Equation, how does the concentration of reactants affect the cell potential?
How does Kohlrausch's Law of independent migration of ions help in electrochemistry?