
Reduction Formula : The reduction formula is a crucial concept in the NEET 2025 syllabus , it involves the addition of electrons or the removal of oxygen or other electronegative elements from a chemical species.
It plays an important role in transforming compounds into more reduced forms, often employing reducing agents like lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4).Reduction Formula for NEET 2025
Reduction reactions are important in various processes, including the conversion of carbonyl compounds to alcohols. These reactions contribute significantly to the synthesis of complex molecules and the creation of diverse chemical structures, making the reduction formula an indispensable tool in the toolkit of synthetic chemists.Lithium Aluminium Hydride – LiAlH4
LiAlH4 is a powerful reducing agent widely used in organic synthesis. It reacts vigorously with alcohols, undergoing hydride transfer to form corresponding alkoxides.
 One significant aspect to note about lithium aluminum hydride is its violent reaction with water.
One significant aspect to note about lithium aluminum hydride is its violent reaction with water. 
Interaction with Alcohols
It reacts with alcohols to produce alkoxides, facilitating subsequent transformations in organic chemistry, such as the reduction of carbonyl compounds.
Reduction of Various Ketones
It is employed in the reduction of ketones, chemically breaking the carbonyl group and leading to the formation of alcohols.
Reduction of Camphor
It reduces camphor to isoborneol, showcasing its utility in complex molecule transformations and synthetic processes.
Sodium Borohydride (NaBH 4 )
NaBH4 is a milder reducing agent compared to LiAlH4, commonly used in reducing aldehydes and ketones.
Reduction of Aldehydes and Ketones
NaBH4 reduces aldehydes and ketones selectively, providing a controlled method for synthesizing various compounds in organic chemistry.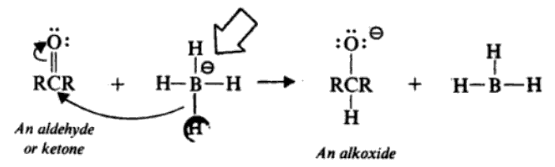
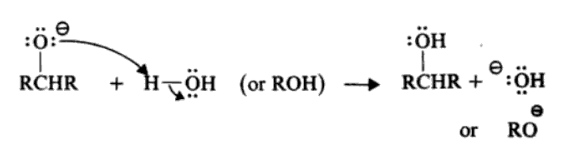
Rosenmund Reduction
A selective reduction process using a palladium catalyst on barium sulfate, converting acyl chlorides to aldehydes.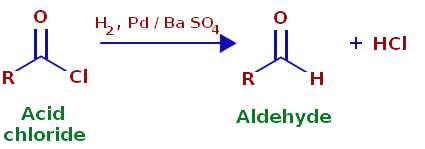
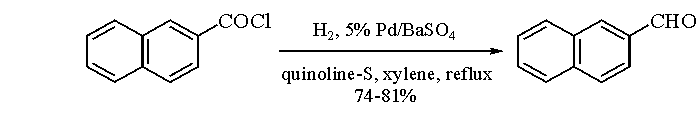
Birch Reduction
Birch reduction involves the use of alkali metals in the presence of ammonia, reducing aromatic compounds to 1,4-cyclohexadiene.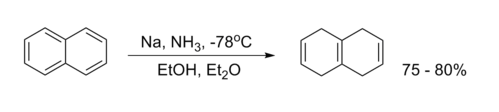
Stephen’s Reduction
Stephen’s reduction utilizes tin(II) chloride to selectively reduce aromatic nitro groups to amino groups.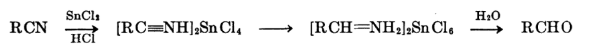
Examples of Stephen’s Reduction
Common examples include the reduction of nitrobenzene to aniline, showcasing the versatility of this synthetic method in producing amines.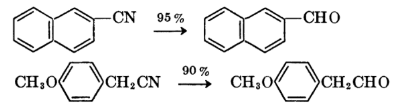
Clemmensen Reduction
A classic reduction method employing zinc amalgam and hydrochloric acid, reducing ketones and aldehydes to alkanes.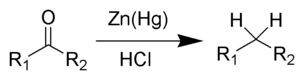
Wolff Kishner Reduction
Wolff-Kishner reduction, a powerful technique, uses hydrazine and a strong base to convert carbonyl groups into corresponding hydrocarbons, avoiding the use of metal hydrides.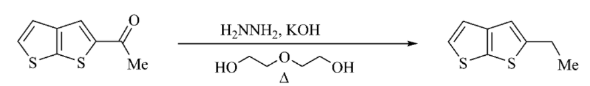 Prepare for NEET with PW's NEET online coaching . We offer multiple online courses that offer dedicated faculty, interactive classes, and in-depth study materials to ace your exams with personalized guidance, regular assessments, and mock tests.
Prepare for NEET with PW's NEET online coaching . We offer multiple online courses that offer dedicated faculty, interactive classes, and in-depth study materials to ace your exams with personalized guidance, regular assessments, and mock tests.|
NEET Important Formulas for 2025 |
|
Reduction Formula FAQs
What is the formula of reduction method?
The reduction method in chemistry doesn't have a single formula, as it contains a variety of reactions involving the gain of electrons or removal of oxygen. Common reducing agents like lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4) are often used, but specific reactions dictate the overall formula.
How do you write a reduction formula?
To write a reduction formula, identify the reactants and products involved in the reduction reaction. Commonly, reducing agents like LiAlH4 or NaBH4 are used, and the reaction specifics depend on the starting material (e.g., ketones, aldehydes). The reduction process typically involves the addition of electrons or removal of oxygen.
How is reduction done in chemistry?
Reduction in chemistry involves reactions where a chemical species gains electrons or undergoes a decrease in oxidation state. This can result in the removal of oxygen, addition of hydrogen, or gaining of electrons from a reducing agent. Reduction reactions are crucial in organic synthesis, transforming functional groups like carbonyls into alcohols.
What are the four types of reduction?
Four Types of Reduction:
Reduction reactions can be classified into four types:
Addition of Hydrogen: Involves the addition of hydrogen to a molecule.
Removal of Oxygen: Involves the removal of oxygen from a compound.
Gain of Electrons: Involves the gain of electrons by a chemical species.
Loss of Oxidation State: Involves a decrease in the oxidation state of an element within a compound.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









