
Grignard Reagent Formula for NEET : The formula for a Grignard reagent is typically represented as RMgX, where R is an alkyl or aryl group, and X is a halogen (commonly Cl, Br, or I). The presence of a magnesium (Mg) atom creates a highly reactive species.
This reagent is crucial in organic synthesis, particularly for forming carbon-carbon bonds, making it an essential topic in the NEET 2025 syllabus syllabus for aspiring medical students. Understanding the Grignard reagent's structure and reactivity is fundamental for success in organic chemistry, offering a versatile tool for various chemical transformations.
Grignard Reagent Formula for NEET 2025
The formula, usually represented as RMgX, involves an alkyl or aryl group (R) combined with a magnesium atom (Mg) and a halogen (X). Grignard reagents play a vital role in organic synthesis, forming carbon-carbon bonds in various reactions.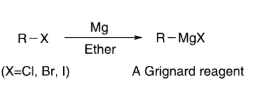 Mastery of this formula is essential for NEET aspirants, enabling them to understand and apply Grignard reagent reactions effectively. It serves as a fundamental building block in the study of organic chemistry, contributing significantly to the detailed preparation required for success in the NEET 2025 exam.
Mastery of this formula is essential for NEET aspirants, enabling them to understand and apply Grignard reagent reactions effectively. It serves as a fundamental building block in the study of organic chemistry, contributing significantly to the detailed preparation required for success in the NEET 2025 exam.
Reactions of Aldehyde and Ketone
The Reactions of Aldehyde and Ketone are fundamental aspects of organic chemistry, crucial for NEET 2025 preparation. Aldehydes and ketones undergo diverse transformations, including nucleophilic addition, oxidation, and reduction. Nucleophilic addition reactions with water, in the presence of acids or bases, form hydrates, influencing the equilibrium of carbonyl compounds.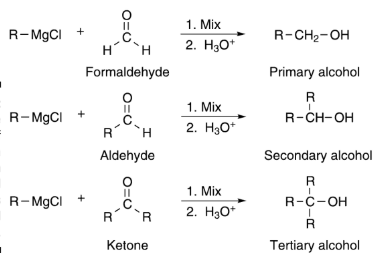
List of Reactions
Below is the list of reactions involved in the Grignard Reagent Formula, you can check it out and build a strong understanding of this topic.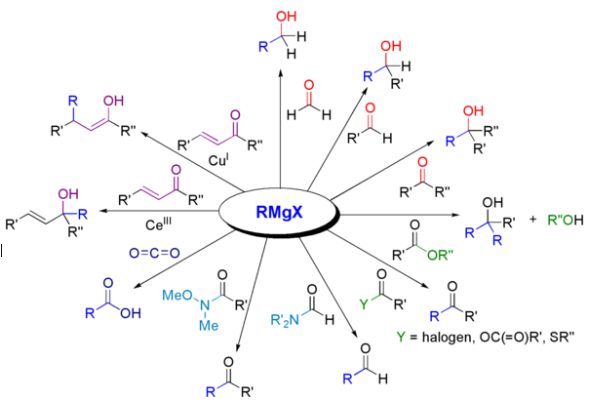
Addition to Carbonyl Compounds
In this reaction, nucleophiles add to the carbon-oxygen double bond in carbonyl compounds, forming new bonds and changing the structure.RMgX + C=O → RCHO (Aldehyde) or R2CHOH (Secondary Alcohol)
Formation of Alcohols
Aldehydes and ketones react with water or other reducing agents, producing alcohols as products, which is a common transformation in organic chemistry.RMgX + H2O → R-OH (Alcohol)
Reaction with Epoxides
Aldehydes and ketones react with epoxides, opening the ring and forming new functional groups in the process.RMgX + Cyclic Ether → Opened Alcohol
Reaction with Nitriles
The reaction involves the addition of Grignard reagents to nitriles, leading to the formation of amides, a crucial process in organic synthesis.RMgX + RC≡N → R-CO-NH2 (Amide)
Reaction with Esters
Aldehydes or ketones react with esters in the presence of a catalyst, resulting in the formation of beta-keto esters, contributing to the synthesis of complex molecules.RMgX + R'-CO-OR'' → R-R' (Ketone)
Halide Exchange (Schlenk Equilibrium)
This equilibrium involves the exchange of halide ions between a Grignard reagent and an alkyl halide, providing a versatile tool for organic transformations.RMgX + R'X' → RR' + MgX'2
Reaction with Acyl Chlorides
Aldehydes and ketones react with acyl chlorides to form compounds known as ketals, essential in various synthetic processes.RMgX + R'-CO-Cl → R-R' (Ketone)
Reduction of Alkynes
Aldehydes and ketones can be produced from the reduction of alkynes, providing an alternative route to these functional groups.RMgX + RC≡CH → R-CH=CH2 (Alkene)
Reaction with Acid Halides
Aldehydes and ketones react with acid halides to form compounds known as hemiacetals, contributing to the synthesis of complex organic molecules.RMgX + R'-CO-X → R-R' (Ketone)
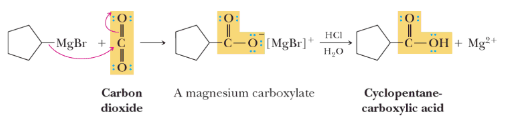
Carboxylation (Reaction with CO2)
In this reaction, Grignard reagents react with carbon dioxide, resulting in the formation of carboxylic acids, which is a significant step in organic synthesis.RMgX + CO2 → R-COO-MgX (Carboxylate)
PW's NEET online coaching offers syllabus-oriented and dedicated preparation for the NEET 2025 exam with expert faculty, interactive sessions, and a personalized approach. Join and access quality study materials, live classes, and mock tests from the comfort of your home.|
NEET Exam 2025 Important Formulas |
|
Grignard Reagent Formula FAQs
What is the Grignard reaction in NEET?
The Grignard reaction is an essential organic chemistry reaction featured in NEET. It involves the addition of an alkyl or aryl magnesium halide (Grignard reagent) to a carbonyl compound, forming a new carbon-carbon bond. This reaction is crucial for synthesizing various organic compounds.
What is the equation for the Grignard reagent in Class 12?
The general equation for the formation of a Grignard reagent (R-MgX) involves the reaction of an alkyl or aryl halide (R-X) with magnesium (Mg) in anhydrous ether or any suitable solvent.
Example: R-X + Mg → R-MgX (in the presence of ether)
Which of the following formula represents the Grignard reagent?
The formula for a generic Grignard reagent is R-MgX, where R represents an alkyl or aryl group, and X is a halogen (commonly Cl, Br, or I).
What is the formula for the reagent MG?
The formula for magnesium, the metal used in the Grignard reaction, is Mg.
What are the 5 applications of Grignard reagents?
Synthesis of Alcohols: Grignard reagents react with carbonyl compounds to form alcohols.
Formation of Hydrocarbons: They are used to prepare hydrocarbons by reacting with alkyl or aryl halides.
Carboxylic Acid Synthesis: Grignard reagents react with carbon dioxide to produce carboxylic acids.
Nitrile Formation: They react with alkyl or aryl halides followed by cyanide ion to form nitriles.
Reduction of Esters: Grignard reagents can reduce esters to form primary alcohols.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









