
Base
Acidic, Basic and Neutral materials of Class 7
Defination of base
Substances with bitter taste and soapy touch are known as bases. There are many bases like NaOH and KOH that have corrosive action on the skin and can even harm the body, so according to the modern definition – Base is defined as a substance capable of releasing one or more OH - ions in aqueous solution.
ALKALIES:
Some bases like NaOH and KOH are water soluble. These are known as alkalies.(bases which are soluble in water) Therefore water soluble bases are known as alkalies eg. KOH, NaOH. A list of a few typical bases along with their chemical formulae and uses is given below-
| Name | Commercial Name | Chemical Formula | Uses |
| Sodium hydroxide | Caustic Soda | NaOH | In manufacture of soap, paper, pulp, rayon, refining of petroleum etc. |
| Potassium hydroxide | Caustic Sba | KHO | In alkaline storage batteries, manufacture of soap, absorbing CO 2 gas etc. |
| Calcium hydroxide | Slaked lime | Ca(OH) 2 | In manufacture of bleaching powder softening of hard water etc. |
| Magnesium hydroxide | Milk of Magnesia | Mg(OH) 2 | As an antacid to remove acidity from stomach |
| Aluminum hydroxide | – | Al(OH) 3 | As foaming agent in fire extinguishers. |
| Ammonium hydroxide | – | NH 4 OH | In removing greases stains from cloths and in cleaning window panes. |
PHYSICAL PROPERTIES OF BASES
- Bitter taste of bases: Almost all basic solution are bitter in taste.
- Action on litmus solution: Red litmus solution is converted into blue by bases
- Action on methyl orange: Bases turn methyl orange into yellow.
- Action on phenolphthalein: Bases turn phenolphthalein into pink.
- Conduction of electricity: Like acid, the aqueous solution of a base also conducts electricity.
CHEMICAL PROPERTIES OF BASES
REACTION OF BASES WITH METALS: Metals like zinc, tin and aluminum react with strong alkalies like NaOH (caustic soda), KOH (caustic potash) to evolve hydrogen gas.
-
Zn(s) + 2NaOH(aq) →Na2ZnO2 (aq) + H
2
(g)
Sodium zincate
-
Sn(s) + 2NaOH(aq) → Na
2
SnO
2
(aq) + H
2
(g)
Sodium stannite
-
2AI(s) + 2NaOH + 2H
2
O → 2NaAIO
2
(aq) + 3H
2
(g)
Sodium meta aluminate
Experiment: If we take a small number of zinc granules in a test tube and add about 2-3 ml of concentrated NaOH solution into it and hear this mixture in low temperature.
Observation: There is evolution of gas which burns with a pop sound (on bringing a burning candle near the mouth of tube).
The reaction involved is:
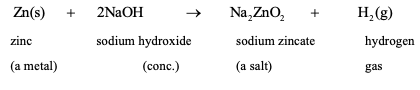
zinc sodium hydroxide sodium zincatehydrogen (a metal) (conc.)(a salt)gas
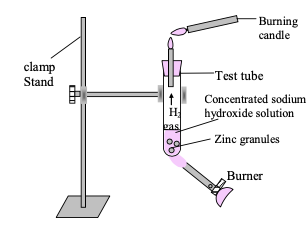
Figure-Study of the reaction of sodium hydroxide with Zn metal







