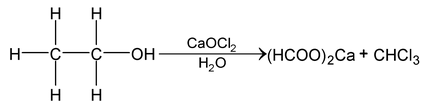
Chemical Properties
Alcohol Phenol And Ether of Class 12
Chemically properties of alcohols can be discussed under following categories:
- Reaction involving breaking of carbon – oxygen bond.
- Reaction involving breaking of oxygen – hydrogen bond.
- Oxidation of alcohols.
- Dehydrogenation of alcohols.
- Some miscellaneous reactions of monohydric alcohol.
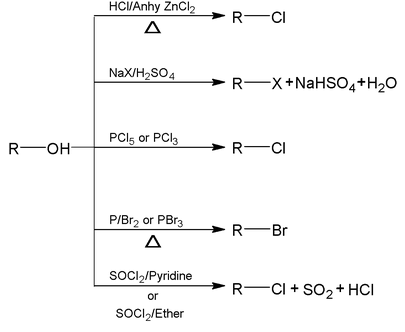
Reaction involving breaking of carbon – oxygen bond
Order of reactivity of alcohol. 3° > 2° > 1°
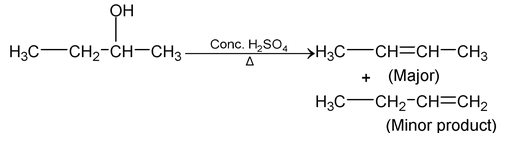
- Dehydration of alcohol
- Dehydration of alcohol to give alkene.Dehydrating agents are Conc H2SO4/Δ, KHSO4/Δ, H3PO4/Δ, Anhy Al2 O3/Δ, Anhy PCl5/Δ, Anhy ZnCl2/Δ, BF3/Δ, P2 O5/Δ.
- Reactivity of alcohols. (Ease of dehydration)
- 3° > 2° > 1°
- Product formation always takes place by saytzeff rule.
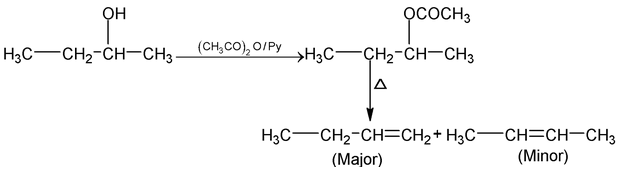
Alcohols on acetylation gives acetyl derivative which on pyrolytic elimination always gives Hofmann product.
- Mechanism in presence of acidic medium
E
1
mechanism: follow saytzeff’s rule.
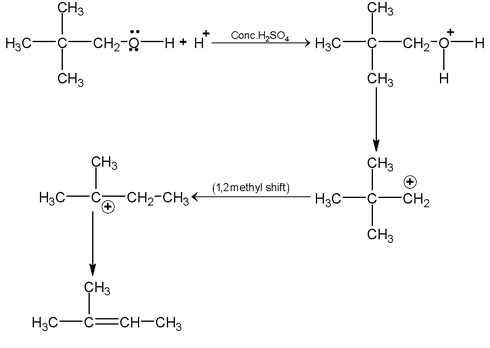
- Reactions due to breaking of oxygen hydrogen bond. (Reactions due to acidic character of alcohols)
Alcohols are acidic in nature because hydrogen is present on electro negative oxygen atom.
Alcohol is weaker acid
 acidity α stability of acid anions.
acidity α stability of acid anions.
Acidity of 1° > 2° > 3°
Alcohols give following reactions due to breaking of oxygen – hydrogen bond.
Reaction with metal
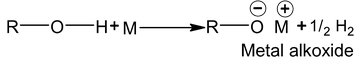
M = 1st group metal.
M = Al, Mg, Zn
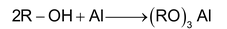
Aluminium alkoxide
Esterification (With carboxylic acid)
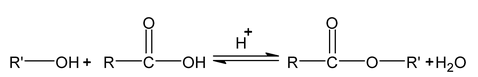
It is reversible acid catalysed reaction. It follow SN
1
mechanism.
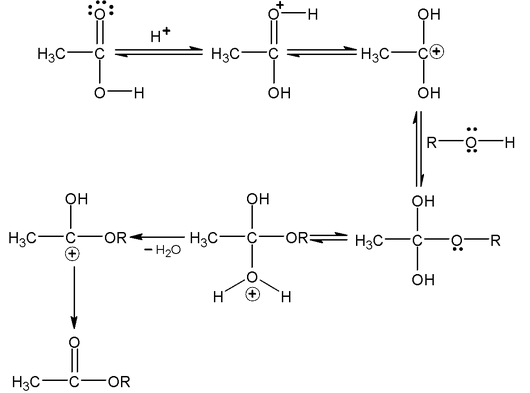
Increasing the size of alkyl group on alcohol part decreases the nucleophilic character because steric hindrance increases.

Order of alcohol CH
3
OH > 1° > 2° alc > 3° alc
Ester formation with proton acid having –OH group: to give inorganic ester.
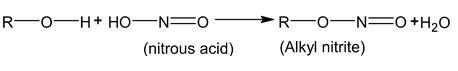
Alkylation of Alcohol
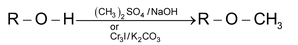
Methylation is mainly used for determination of hydroxyl groups in an unknown compound.

Oxidation of alcohol is dehydrogenation reaction which is 1, 2 – elimination reaction.
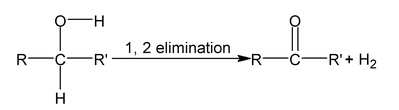
So oxidation of alcohol α numbers of α - hydrogen atom.
With mild oxidising agents:Like
X
2
Fenton reagent [FeSO
4
/H
2
O
2
].
Jones reagent / CH
3
COCH
3
[CrO
3
/dil. BaSO
4
].
K
2
Cr
2
O
7
/H
+
cold
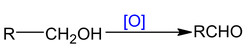
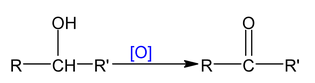
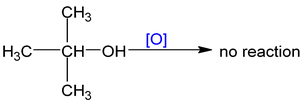
Note: PCC (Pyridinium chloro chromate) is a selective reagent which converts 1° alc to aldehyde.
With strong oxidising agent Oxidising agents are
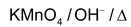
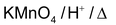
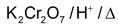

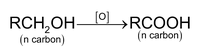
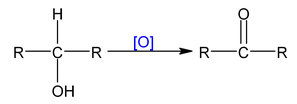
Dehydrogenation with Cu/573K or Ag/573K
1° alcohol ⎯⎯→ aldehyde
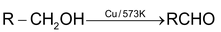
2° alcohol ⎯⎯→ ketone
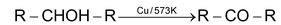
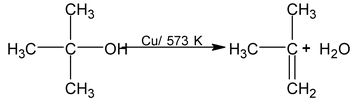
3° alc ⎯→ undergo dehydration to form alkene.

Miscellaneous reactions of mono hydric alcohol
Methylation with CH
2
N
2
in presence of BF
3
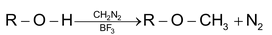
Ethyl alcohol and 2° methyl alcohol gives haloform reaction.
Distinguishing 1°, 2°, 3° alcohol
| Test | 1° alc | 2° alc | 3° alc |
|---|---|---|---|
| (I) Lucas test [ZnCl + HCl] | No reaction at room temperature |
White turbidity after 5 – 10 min.
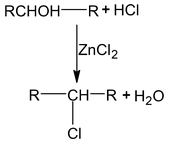
|
While turbidity instantaneously
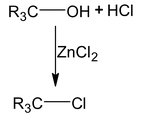
|
| (II) Victor Meyer test (P/I 2 , AgNO 2 , HNO 2 , NaOH) | Red colour | Blue colour |
Colourless
|
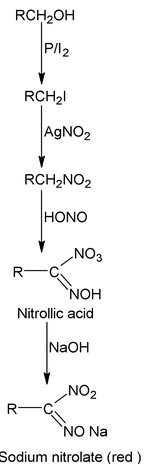
|
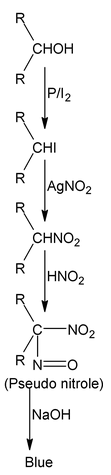
|
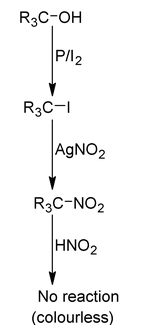
|
Periodate oxidation
Compounds that have hydroxyl group on adjacent atoms undergo oxidation cleavage when they are treated with aq. Periodic acid (HIO 4 ). The reaction breaks carbon carbon bonds and produced carbonyl compounds (aldehyde, ketones or acids)
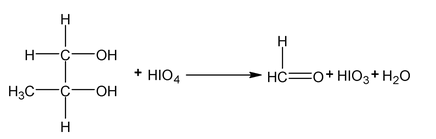
It takes place through a cyclic intermediate.
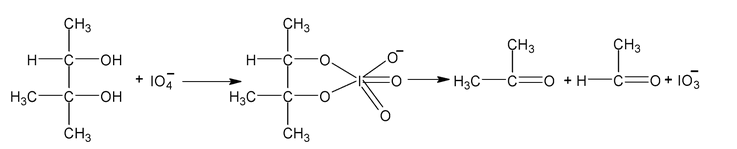
Other examples
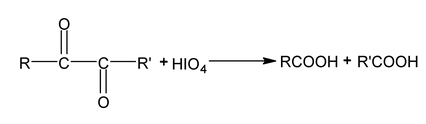
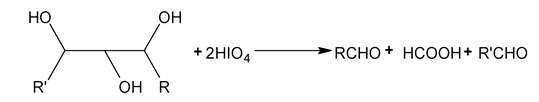
This oxidation is useful in determination of structure.
Pinacol – pinecone rearrangement
Action of H 2 SO 4 on 1, 2 diols.
Ditertiory – 1, 2 diols convert in to ketones on treatment with H 2 SO 4
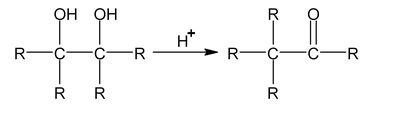
Mechanism
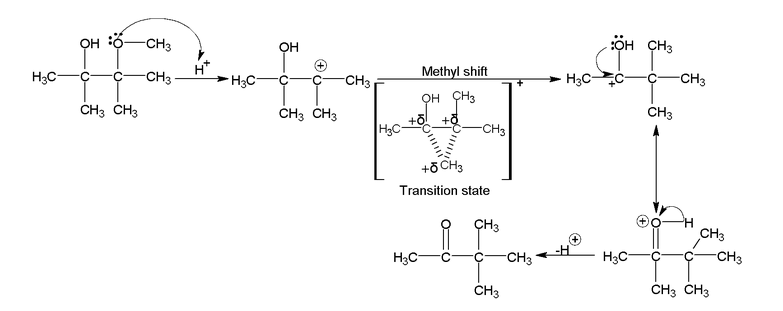
Migratory preference of the group
Migration depends on the stability of Transition state.
In general migration of C 6 H 5 > alkyl
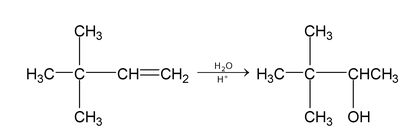
Hydration:
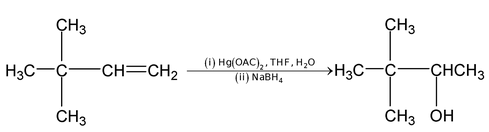
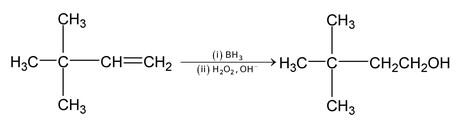
Hydroboration
Oxymercuration – demercuration