
Nomenclature Of Amines
Amines of Class 12
Nomenclature of amines is quite simple. Aliphatic amines are named by naming the alkyl group (or) groups attached to nitrogen , and following that by the word amine.
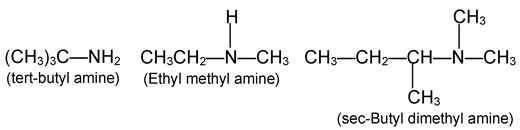
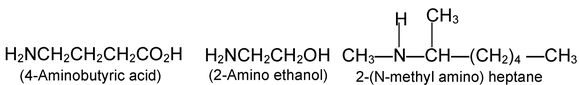
More complicated amines are often named as prefixing amino - (or-N-methylamino -, N-N, diethyl amino -, etc) to the name of the parent chain.
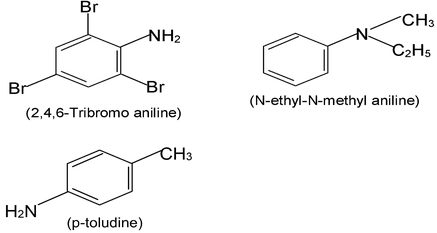
Aromatic amines - those in which nitrogen is attached to an aromatic ring - are generally named as derivatives of the simplest aromatic amine, aniline.
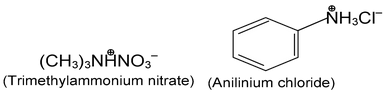
Salts of amines are generally named by replacing - amine by - ammonium (or - aniline by - anilinium), and adding the name of the anion.
Physical Properties
Amines are moderately polar substances; they have boiling points that are higher than those of alkanes but generally lower than alcohols of comparable molecular weight. Molecules of primary and secondary amines can form strong hydrogen bonds to each other and to water. Molecules of tertiary amines can not form hydrogen bonds to each other, but they can form hydrogen bonds to molecules of water or other hydroxylic solvents. As a result, tertiary amines generally boil at lower temperatures than primary and secondary amines of comparable molecular weight.







