carbon and its compound
Carbon And Its Compound of Class 10
Carbon belongs to the 14 th group of the periodic table. Carbon forms organic as well as inorganic compounds.
Originally compounds like urea, sugars, fats, oils, dyes, proteins, vitamins etc, which were isolated directly or indirectly from living organisms such as animals and plants were called organic compounds.
In contrast, compounds like common salt, marble, alums, nitre, blue and green vitriols etc. which are isolated from non−living sources such as rocks and minerals were called inorganic compounds. Carbon shows some characteristics properties. These are as follows.
Allotropy
The phenomenon of existence of an element in two or more forms which have different physical properties but identical chemical properties is called allotropy and the different forms are called allotropic forms or simply allotropes.
Carbon occurs in three crystalline allotropic forms. These are :
- Diamond
- Graphite
- Fullerenes
DIAMOND
Occurrence: Although diamonds occur in nature, They have also been synthesized by subjecting pure carbon to very high pressure (50,000 – 60,000 atmospheres) and high temperature (1873 K). The synthetic diamonds are small in size but are otherwise indistinguishable from natural diamonds.
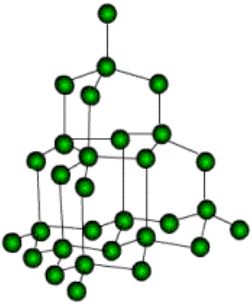
Structure of Diamond
Structure of Diamond:
Diamond crystals found in nature are generally octahedral (eight faced). In the structure of diamond, each carbon is linked to four other carbon atoms forming a regular and tetrahedral arrangement and this network of carbon atoms extends in three dimensions and is very rigid. This strong bonding is the cause of its hardness and its high density. This regular, symmetrical arrangement makes the structure very difficult to break. To separate one carbon atom from the structure, we have to break four strong covalent bonds.
Elements in which atoms are bonded covalently found in solid state.
e.g. diamond, graphite, sulphur etc.
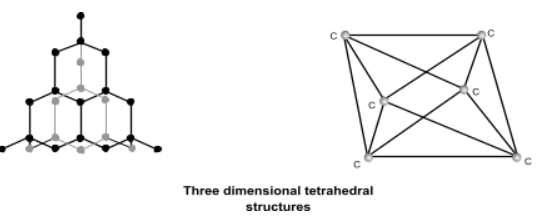
Physical Properties:
- Hardness: The three-dimensional network structure of diamond makes it the hardest natural substance known. It is because of this hardness, diamond is used for drilling, grinding and polishing equipments like rock borers, glass cutters, dies, etc.
- High density: Due to network structure, the carbon atoms in diamond are closely packed and hence diamond has a high density.
- High melting point: A large amount of energy is needed to break the network structure of diamond. Therefore, the melting point of diamond is quite high (3930°C or 4203 K).
- Electrical and Thermal conductivity: Since all the four valence electrons are firmly held in carbon-carbon single bonds, there are no free electrons in a diamond crystal. Therefore, diamond is a bad conductor of electricity.
- Transparency: Because of high refractive index (2.42), diamonds can reflect and refract light. As a result, diamonds are transparent substances.
Uses of Diamond:
- They are used in jewellery because of their ability to reflect and refract light.
- Diamonds are used in cutting glass and drilling rocks.
- Diamond has an extraordinary sensitivity to hot rays and due to this reason, it is used for making high precision thermometers.
- Diamond has the ability to cut out harmful radiations and due to this reason it is used for making protective windows for space probes.
- Diamond dies are used for drawing thin wires. Very thin tungsten wires of diameter less than one-sixth of the diameter of human hair have been drawn using diamond dies.
- Surgeons use diamond knives for performing delicate operations.