Rate Of Reaction
Chemical Kinetics of Class 12
The rate of a reaction means the speed with which the reaction takes place. This is expressed either in terms of decrease in the concentration of a reactant per unit time or increase in the concentration of a product per unit time.
Rate of reaction

Or,

The term
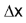 means
means
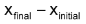 and
and
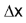 is the amount of time elapsed. For example, a car driver starts his journey at 9.00 AM with odometer reading x miles. At 11.00 AM, he reaches his destination. The odometer reading at destination is y miles. The rate of his travel can be calculated as
is the amount of time elapsed. For example, a car driver starts his journey at 9.00 AM with odometer reading x miles. At 11.00 AM, he reaches his destination. The odometer reading at destination is y miles. The rate of his travel can be calculated as
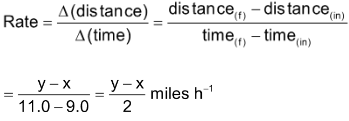
The above example indicates that the car has been driven with uniform rate but actually it has been driven sometimes faster and sometimes slower depending upon the condition of road. Thus, the overall rate is an average rate and the rate at which the car was moving at any instan
t, called instantaneous rate. The rate measured over a long time interval is called average rate and the rate measured for an infinitesimally small time interval is called instantaneous rate.
In general, for any reaction of the type

Average rate of reaction
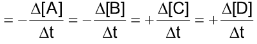
Where [A] signifies the molar conc. of (A) and
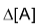 stands for the change in molar concentration of A. The negative sign placed before a reaction rate symbol signifies a decrease in concentration of the reactant with increase of time and a positive sign before the rate symbol signifies that the concentration of product increases with increase of time.
stands for the change in molar concentration of A. The negative sign placed before a reaction rate symbol signifies a decrease in concentration of the reactant with increase of time and a positive sign before the rate symbol signifies that the concentration of product increases with increase of time.
Average rate of reaction
The rate measured over a long time interval is called average rate. The rate of reaction (average rate) is defined as the change of concentration of any one of the reactants (or products) per unit time.
Average rate of reaction

Consider the reaction between CO and

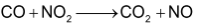
This equation shows that one mole of CO reacts with one mole of
 one mole each of
one mole each of
 are formed. The average rate of reaction can be expressed either by decrease in conc. of reactant
are formed. The average rate of reaction can be expressed either by decrease in conc. of reactant
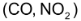 or by the increase in conc. of any one of products
or by the increase in conc. of any one of products
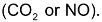
Thus,
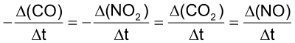
For the reaction,
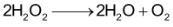
When 2 moles of
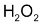 decomposed, one mole of
decomposed, one mole of
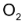 and 2 moles of
and 2 moles of
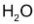 is formed. The rate of increase in the conc. of
is formed. The rate of increase in the conc. of
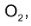 therefore is half that of the disappearance of the conc. of
therefore is half that of the disappearance of the conc. of
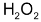 and increase in conc. of
and increase in conc. of
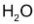 is the same of the disappearance of the conc. of
is the same of the disappearance of the conc. of
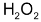 in the same time interval.
in the same time interval.
So
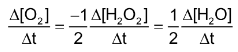
In general, for a reaction,

The rate is expressed as:
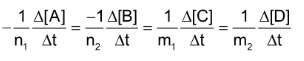
Instantaneous rate
|
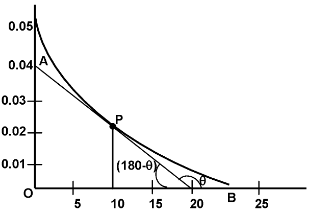
With the progress of reaction the conc. of reactants decreases while that of product increases. According to law of mass action the rate of reaction decreases moment to moment as shown by graph of rate vs. time. Rate varies from moment to moment so rate of reaction has to be specified at a given instant of time called instantaneous rate
|
|
Where dC is the infinitesimal change in conc. during infinitesimal time interval dt after time t i.e. between t and t + dt.
|
Consider a reaction
|
|
Thus the rate of reaction at time 10 minutes

Units of the rate of reaction
Since concentration is usually expressed in moles / time and time is taken in seconds or minutes, the unit of the rate of reaction is moles
 or
or
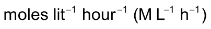 or moles
or moles


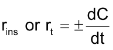
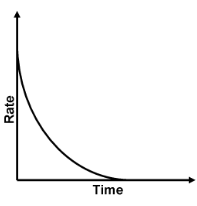
 ,To know the rate of reaction at any time t, a tangent is drawn to curve at the point corresponding to that time and it is extended on either side so as to cut the axes, say at the point A and B. Then
,To know the rate of reaction at any time t, a tangent is drawn to curve at the point corresponding to that time and it is extended on either side so as to cut the axes, say at the point A and B. Then