
Electron Affinity
Periodic Properties of Class 11
The amount of energy released when an electron is added to the outermost shell of one mole of an isolated gaseous atom in its lower energy state is called electron affinity. Electron affinity just defined is actually first electron affinity since it corresponds to the addition of one electron only. In the process of adding further electron, the second electron will be added to gaseous anion against the electrostatic repulsion between the electron being added and the gaseous anion. Sometimes energy instead of being released is supplied for the addition of an electron to an anion.
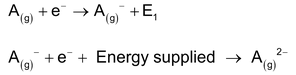
Factors Affecting the Magnitude of Electron Affinity:
- Atomic size: In general electron affinity value decreases with the increasing atomic radius because electrostatic force of attraction decreases between the electron being added and the atomic nucleus due to increase of distance between them.
Electron affinity α 1/Atomic size
- Effective nuclear charge: Electron affinity value of the element increase as the effective nuclear charge on the atomic nucleus increases because electrostatic force of attraction between the electrons being added and the nucleus increases. As the electrostatic force of attraction increases, amount of energy released is more.
Electron affinity α Effective nuclear charge (Zeff)
-
Screening or Shielding effect: Electron affinity value of the elements decreases with the increasing shielding or screening effect. The shielding effect between the outer electrons and the nucleus increases as the number of electrons increases in the inner shells. Electron affinity α 1/Shielding effect
- Stability of half filled and completely filled orbitals: The stability of half filled and completely filled degenerate orbitals of a sub shell is comparatively more, so it is difficult to add electron in such orbitals and lesser energy is released on addition of electrons hence the electron affinity value will decrease.
PERIODICITY IN ELECTRON AFFINITY
- In general electron affinity value increases in moving from left to right in a period because effective nuclear charge increases.
Exceptions:
- The electron affinity value of alkaline earth metals of IIA group is zero. (only Be and mg)
- Electron affinity value of alkali metals of IA group is also approximately zero because these elements have the tendency of losing the electron instead of gaining the electron.
- Electron affinity values of nitrogen and phosphorus (VA) are lesser than the electron affinity values of carbon and silicon respectively. It is due to the comparatively stable half filled configuration (np3) of nitrogen and phosphorus and the tendency to acquire the stable np3 configuration by the gain of one electron in carbon and silicon np2 orbitals.
- The theoretical value of the electron affinity of zero group inert gas elements is zero due to stable s2p6 configuration.
- In a group moving from top to bottom the electron affinity value of elements decreases because the atomic size increases
Exceptions:
-
Electron affinity values of second period elements are smaller than the electron affinity values of third period elements. This unexpected behaviour can be explained by the very much high value of charge densities, (which is equal to
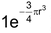 ) of second period elements due to much smaller size. The electron being added experiences comparatively more repulsion and the electron affinity value decreases.
) of second period elements due to much smaller size. The electron being added experiences comparatively more repulsion and the electron affinity value decreases.
- The electron affinity of fluorine (Second period) is less than the electron affinity of chlorine (third period). 2p-orbitals in fluorine are much more compact than 3p- orbitals of chlorine. So the electron being added in 2p-orbitals experiences comparatively more repulsion and the electron affinity value decreases.









