Aromaticity
GOC of Class 11
AROMATICITY :
This phenomenon helps us to predict that a given compound would be aromatic or not. In general, a aromatic compound is the one which has a characteristic aroma (odour). But in chemical practice, if a compound is provided to us (not physically), then how can we judge that the given compound is aromatic or non-aromatic by looking at the structure of the compound. This phenomenon of aromaticity helps us to decide that a given compound would be aromatic or not by observing its structure. For a compound to be aromatic, it should follow the given conditions.
(a) There must be cyclic delocalization in the compound.
(b) The closed loop formed due to cyclic delocalization must have a total of (4n + 2)π electrons where n is an integer having values 0, 1, 2, 3…… The (4n + 2)π electrons is called Hukel number.
The first condition of cyclic delocalization takes into account the conditions required to show resonance i.e. the compound must have conjugation and the compound must be planar or nearly planar.
If both the above conditions are fulfilled, then the compound would be aromatic and if any of the above condition fails, then it is non-aromatic.
First of all, let us take the simplest example of benzene
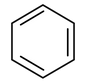
As it is evident from the structure that there is conjugation in the cyclic ring leading to cyclic delocalization as well as the closed loop formed would have 6π electrons (corresponding to
n = 1). Thus, benzene is said to be aromatic compound. The same would be true for phenol and nitrobenzene also.
Now, let us consider the case of naphthalene (I) & anthracene (II)
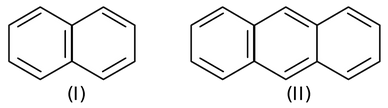
Both these compounds have conjugation in carbocyclic ring leading to cyclic delocalization and the closed loop formed would have 10π and 14π electrons respectively. So, both the compounds are aromatic as both the conditions are fulfilled.
If we are provided with four compounds/species, which are shown as
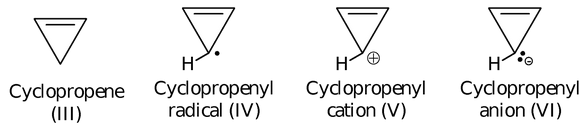
Of these four compounds/species, (III), (IV) and (VI) are non-aromatic, due to the failure of first condition, second condition and second condition respectively. While the species (V) is aromatic as it will have permitted cyclic delocalization and closed π-orbital is having 2π electrons corresponding to n = 0.
If we are provided with following species
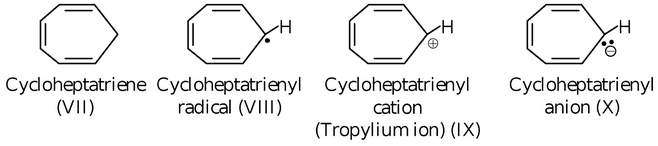
Of the given compounds/species, only species (IX) is aromatic since it satisfies both the conditions required by a compound/species to be aromatic, while (VII), (VIII) and (X) are non−aromatic.
(VII) is non−aromatic since it does not undergo cyclic delocalization.
(VIII) is non−aromatic since in the closed loop it has 7π electrons which is not a Huckel number.
(X) is non−aromatic since in the closed loop it has 8 π electrons which is also not a Huckel number.
Now, let us see whether pyrrole and pyridine are aromatic or not.
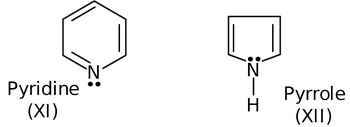
In pyridine, the nitrogen atom is sp 2 hybridized with one of the sp 2 hybrid orbital having a lone pair of electrons. Thus, the sp 2 hybrid lobe with lone pair is out of the plane with respect to the pz orbital of adjacent carbon (as they are at 90° angle), so the lone pair of nitrogen is not involved in delocalization. Thus, cyclic delocalization involves only π-bonding electrons which are six in number. Thus pyridine is aromatic with no involvement of lone pair in cyclic delocalization. While in pyrrole, the nitrogen atom is sp 3 hybridized with one of the sp 3 hybridized orbital having a lone pair of electrons. This sp 3 lobe with lone pair is nearly planar with respect to pz - orbital of the adjacent carbon, so the lone pair of nitrogen is involved in cyclic delocalization and the closed loop will have 6π-electrons. Thus, pyrrole is aromatic in nature.
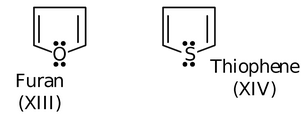
Due to same reasoning, as applied to pyrrole is also applicable to furan (IX) and thiophene(X).