
SEPARATION OF A MIXTURE
Is matter around us pure of Class 9
SEPARATION OF A MIXTURE
Different methods or techniques are used to separate the individual components of a mixture, whether homogeneous or heterogeneous. There can be three cases :
- mixture of two solids
- mixture of a solid and a liquid
- mixture of two liquids
SEPARATION OF A MIXTURE OF TWO SOLIDS:
All the mixtures containing two solid substances can be separated by one of the following methods:
- By using a suitable solvent
- By the process of sublimation
- By using a magnet
Separation by a Suitable Solvent:
In some cases, one constituent of a mixture is soluble in a particular liquid solvent whereas the other constituent is insoluble in it. This difference in the solubilities of the constituents of a mixture can be used to separate them. For example, sugar is soluble in water whereas sand is insoluble in it, so a mixture of sugar and sand can be separated by mixing them in water as solvent.
Separation by Sublimation
The change of a solid directly into vapours on heating, and of vapours into solid on cooling is called sublimation. The process of sublimation is used to separate those substances from a mixture which sublime on heating. The substances like ammonium chloride, iodine, camphor, naphthalene and anthracene sublime on heating and can be recovered in the form of a sublimate by cooling their vapours. Let us perform an activity to separate a mixture of salt and ammonium chloride by sublimation.
Ammonium chloride (NH 4 Cl) undergoes sublimation whereas salt (NaCl) does not undergo sublimation. Hence, on heating in a china dish, only ammonium chloride changes directly from solid to gaseous state and thus its vapours are deposited on the stem of the inverted funnel due to cooling as shown in figure. Pure NH 4 Cl is, thus separated by sublimation whereas, the salt (NaCl) is left behind in the China dish.
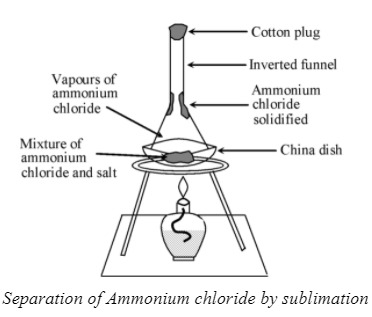
Separation of Ammonium chloride by sublimation
Separation by a Magnet:
Iron is attracted by a magnet. This property of iron is used to separate it from a mixture. So, if a mixture contains iron as one of the constituents, it can be separated by using a magnet. For example, a mixture of iron filings and sulphur powder can be separated by using a magnet. This is because iron filings are attracted by a magnet (and stick to it), but sulphur is not attracted by a magnet.
SEPARATION OF A MIXTURE OF A SOLID AND A LIQUID:
All the mixtures containing a solid and a liquid are separated by one of the following processes:
- By filtration
- By centrifugation
- By evaporation
- By crystallization
- By chromatography
Separation by Filtration:
The process of removing insoluble solids from a liquid by using a filter paper is known as filtration. Filtration is used for separating insoluble substances from a liquid. A mixture of chalk and water is separated by filtration. A mixture of sand and water can also be separated by filtration. The tea-leaves are separated from prepared tea by the method of filtration.
Separation by Centrifugation:
The method of separating finely suspended particles in a liquid, by whirling the liquid at a very high speed is called centrifugation.
- Separation of cream from milk : The process of centrifuging in employed in separating cream from milk. This process is generally employed in separating colloidal solutions which easily pass through the filter paper.
- Principle of centrifugation : It is based on the principle that when a very fine suspension or a colloidal solution is whirled rapidly, then the heavier particles are forced towards the bottom of liquid and the lighter stay at the top.
Method :
- Pour full cream milk in the test tube with a pivot in your laboratory centrifuge.
- Shut the lid of the centrifuge and switch on the current. When the centrifuge starts working, the tub containing milk swings out in the horizontal position and whirls around its axis at a high speed.
- The centrifuge pull (the outward pull) pushes the heavier particles outward, i.e., towards the bottom of the mixture. Thus, the heavier particles of the proteins, carbohydrates, etc. are pushed towards the bottom of the tube, but the lighter particles of the fat stay near the top of the tube and hence separate.
Applications of centrifugation:
- It is employed in milk dairies to separate cream from the milk.
- It is employed in diagnostic laboratories in testing urine samples.
- It is employed in blood banks to separate different constituents of blood.
- It is used in drying machines to squeeze out water from the wet clothes.
Separation by Evaporation:
By Evaporation :
- Separation of coloured components (dye) from blue black ink : The process of evaporation is suitable for the separation of non-volatile soluble solid (dye) from its liquid solvent (water).
Method :
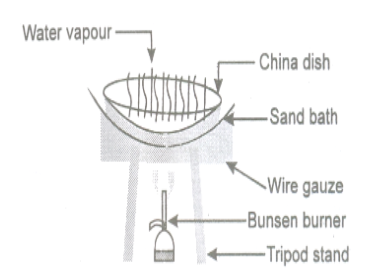
- Heat sand in an iron vessel by placing it over a tripod stand. This arrangement is called sand bath.
- Place a china dish on the sand bath. Pour about 5 cc of the ink into the china dish.
- Heat gently evaporates water from the ink such that it does not boil. In a few minutes the water evaporates leaving behind dry blue black ink. Method of evaporation is suitable for the following solid-liquid mixtures.
|
Solid liquid Mixture |
Non-volatile Solid |
Liquid |
|
Common salt and water |
Common salt |
Water |
|
Sodium nitrate and water |
Sodium nitrate |
Water |
|
Copper sulphate and water |
Copper sulphate |
Water |
Purification by Crystallisation:
The process of cooling hot, concentrated solution of a substance to obtain crystals (purest form of a substance having definite geometrical shapes) is called crystallization. Let us perform an activity to obtain pure copper sulphate from an impure sample by crystallization method.
Procedure: Take about 5g of impure sample of copper sulphate and dissolve it in minimum amount of water in a china dish to make copper sulphate solution. Filter the impurities out. Heat the copper sulphate solution gently on water bath to evaporate water and obtain a saturated solution. Then stop heating. Allow the hot, saturated solution of copper sulphate to cool slowly, for a day.
Observation and Discussion: Crystals of pure copper sulphate are formed by leaving the impurities behind in the solution. This is because, when a saturated solution of copper sulphate is allowed to cool, crystals of copper sulphate separate out. All these crystals have a definite shape and hence look alike. We can separate these crystals from the liquid by the process of filtration and then dry.
Conclusion: When a saturated solution of a substance is allowed to cool, crystals of the substance having definite geometrical shapes separate out.
Application of crystallization: Crystallization can be used to purify :
- Salt that we get from sea water.
- Alum (Phitkari), copper sulphate, nitre (potassium nitrate) etc., from impure samples.
Advantages of crystallization over evaporation:
Crystallization is a better technique than ‘evaporation to dryness’ because of the following reasons:
- Some solids (like sugar) decompose or get charred on heating to dryness during evaporation.
- The soluble impurities do not get removed in the process of evaporation. But such impurities get removed in crystallization.
Separation by Chromatography
Chromatography is a technique of separating two (or more) dissolved solids which are present in a solution in very small quantities. Let us perform an activity to obtain different dyes from black ink by chromatography.
The dye in the black ink is not a single colour, but it is a mixture of 2 or 3 colours. In other words, black ink is a mixture of different colour dyes (solute) in water (solvent), which can be easily identified with the help of paper chromatography.
Paper chromatography is the technique used for separation of those solutes that dissolve in same solvent. The separation by this technique is based upon the principle that though, two (or more) solutes are soluble in the same solvent, but their solubilities may be different.
Procedure : Take a thin strip of filter paper. Draw a line on it with a pencil nearly 3cm above the lower edge. Put a small drop of black ink with the help of pen at the centre of line and let it dry. Suspend the filter paper into a glass containing water so that spot of ink on the paper is just above the water level (figure). Leave it undisturbed for some time.
Observation : The water gradually rises up the filter paper by capillary action and different coloured dyes present in the ink get separated as shown in figure.
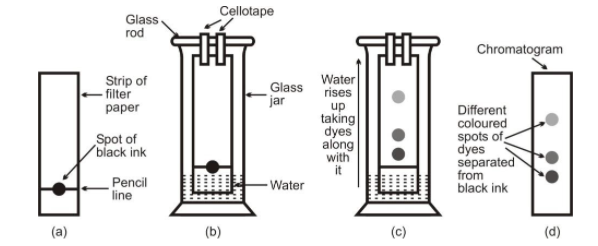
Separation of different coloured dyes by chromatography
Discussion : As water rises up the filter paper, it takes along with it dye particles present in ink. Since, ink is a mixture of two or more coloured dyes, the dye which is more soluble in water, rises faster and produces a coloured spot on the paper at a higher position.
The less soluble dyes present dissolve a little later, rise slower and form coloured spots at lower heights. In this way all the dyes present in black ink get separated (by forming separate coloured spots).
Conclusion : Separation of different coloured dyes present in black ink occurs on a chromatographic paper due to their different solubilities in water.
Hence, from above experiment we conclude that, the dye in black ink is not a single colour, but a mixture of 2 or 3 colours.
|
Application of chromatography:
- Separation of coloured substances present in dyes.
- Separation of pigments from natural colours.
- Separation of drugs from blood.
- Monitoring the progress of a reaction.
- Separation of amino acids obtained by hydrolysis of proteins.
SEPARATION OF A MIXTURE OF TWO (OR MORE) LIQUIDS:
All the mixtures containing two (or more) liquids can be separated by one of the following two methods :
- By the process of distillation.
- By using a separating funnel.
Separation by Distillation
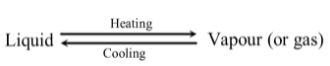
Distillation is the process of heating a liquid to form vapour, and then cooling the vapour to get back liquid. Distillation can be represented as :
The distillation method is of two types:
- Simple distillation method
- Fractional distillation method
Separation of a mixture of miscible liquids is done either by simple distillation method or by fractional distillation method.
|
|
Those liquids which mix together in all proportions and form a single layer (when put in a container), are called miscible liquids. Alcohol and water are miscible liquids because they mix together in all proportions and form a single layer on mixing (The scientific name of common alcohol is ethanol). Water and acetone are also miscible liquids (The scientific name of acetone is propanone). Those liquids which do not mix with each other and form separate layers (when put in a container), are called immiscible liquids. Oil and water are immiscible liquids because they do not mix with each other, and form separate layers on mixing (oil can be mustard oil, groundnut oil, kerosene oil, etc). Petrol and water are also immiscible liquids. |
(a) Separation of a mixture of two miscible liquids by simple distillation method
Simple distillation method is used for the separation of components of a mixture containing two or more miscible liquids which boil without decomposition and have sufficient difference (30–50K) in their boiling points. Let us perform an activity to separate a mixture of two miscible liquids like acetone (B.P. 329 K) and water (B.P. 273K) by simple distillation method.
Procedure : Take the miscible mixture of acetone and water in a distillation flask. Fit it with a thermometer. Arrange the apparatus as shown in figure. Heat the mixture gently noticing the temperature in the thermometer.
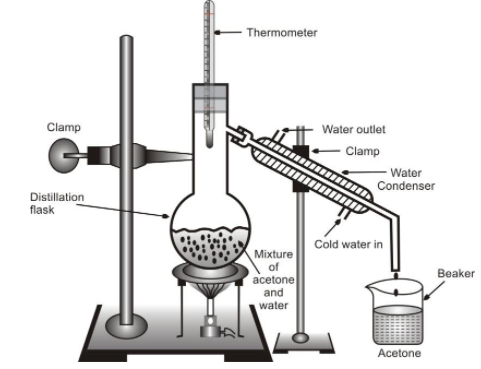
Separation by simple distillation method
Observation : Acetone vaporizes, condenses in the condenser and can be collected from the condenser outlet. Water is left behind in the distillation flask.
Discussion : When the mixture is heated, the vapours of substance having the low boiling point i.e. vapours of acetone are first formed. These travel upwards. On passing through the condenser, they get condensed to form liquid acetone (called distillate) which is collected in the beaker.
|
Conclusion : Separation of the components of a mixture containing two miscible liquids which boil without decomposition and have sufficient difference (30-50 K) in their boiling points can be separated out by simple distillation. This is because at the boiling point of each liquid, the vapours almost entirely consist of that liquid.
Application of simple distillation
The technique of distillation can be used to separate:
- a mixture of ether (b.p. 308 K) and toluene (b.p. 384 K).
- a mixture of hexane (b.p. 342 K) and toluene (b.p. 384 K).
- a mixture of benzene (b.p. 353 K) and aniline (b.p. 457 K) or nitrobenzene (b.p. 483 K)
(b) Separation of mixture of two or more miscible liquids by fractional distillation
Fractional distillation is the process of separating two (or more) miscible liquids by distillation, the distillate being collected in fractions, boiling at different temperatures. The separation of two liquids by fractional distillation depends on the difference in their boiling points. Fractional distillation is carried out by using a fractionating column.
Similarly, different gases from air and different fractions (like kerosene, petrol and diesel etc.) from petroleum products, are also separated by fractional distillation.
Fractional distillation is carried out using a fractionating column which is fitted in between the distillation flask and the condenser as shown in figure. Fractionating column is a tube packed with glass beads. The beads provide surface for the vapours to cool and condense repeatedly.
|
|
The actual purpose of the fractionating column is to increase the cooling surface area and to provide hurdles or obstructions to the ascending vapours and descending liquids. |
(i) Let us perform an activity to separate a mixture of two miscible liquids alcohol and water by Fractional distillation.
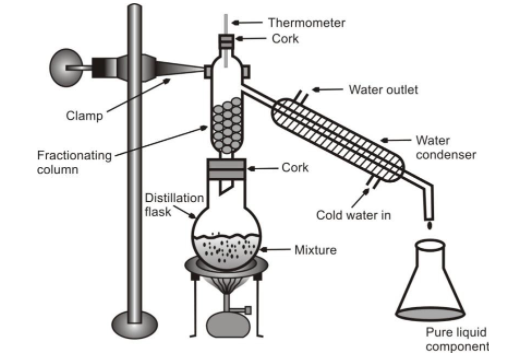
Separation by fractional distillation method
Alcohol (or ethanol), and water are miscible liquids. The boiling point of alcohol is 78 0 C and the boiling point of water is 100 0 C. Since the boiling points of alcohol and water are different, therefore, a mixture of alcohol and water can be separated by fractional distillation. The apparatus used for fractional distillation of alcohol and water mixture is shown in figure.
The mixture of alcohol and water is heated in a distillation flask fitted with a fractionating column. When the mixture is heated, both alcohol and water form vapours as their boiling points approach. The alcohol vapour and water vapour rise up in the fractionating column. The upper part of the fractionating column is cooler, so as the hot vapours rise up in the column, they get cooled, condense and trickle back into the distillation flask. As the experiment goes on, the fractionating column warms up due to the heat released by the condensed vapours. After some time, a temperature gradient is created in the fractionating column, the temperature at the top of the column being much less than at its bottom.
When the temperature at the top of the fractionating column reaches 78 0 C (which is the boiling point of alcohol), then alcohol vapour passes into the condenser, gets cooled and collects in a beaker kept at the other end of the condenser. In this way, all the alcohol distils over and gets separated. It is collected as the first fraction.
Having collected the alcohol fraction, the flask is heated more strongly so that the thermometer shows a temperature of 100 0 C, which is the boiling point of water. When the temperature at the top of the fractionating column becomes 100 0 C, water vapour passes into the condenser, gets cooled and condenses. This pure water is collected in another beaker as the second fraction. Heating is continued till all the water distils over. In this way, the alcohol-water mixture has been separated into two fractions boiling at 78 0 C and 100 0 C, respectively.
|
|
Fractional distillation separates the various liquids according to their boiling points : the more volatile liquid (having lower boiling point) distils over first, and the less volatile liquid (having higher boiling point) distils over later. |
(ii) We can obtain different gases from air by fractional distillation.
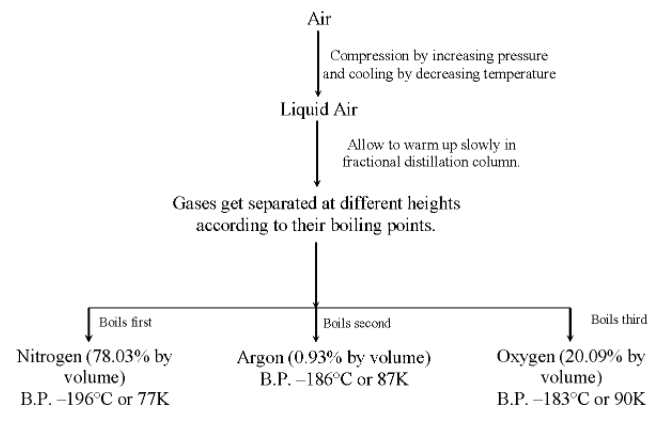
Air is a Homogeneous mixture of a number of gases such as nitrogen, oxygen, argon, carbon dioxide, helium, neon, krypton and xenon. The major components of air are : nitrogen (78.03%), oxygen (20.09%) and argon (0.93%). All the remaining gases of air constitute only 0.05%.
The various gases of air are separated from one another by the fractional distillation of liquid air. For this purpose, air is first compressed by increasing the pressure and then cooled by decreasing the temperature to get liquid air (liquid air is an extremely cold liquid which contains all the component gases in liquid form). The liquid air is then subjected to fractional distillation (or allowed to warm up slowly in a fractional distillation column). As a result, the various liquified gases present in it, boil off at different temperatures (according to their boiling points) and collected separately at different heights in the fractional distillation column.
Since the boiling point of nitrogen is lowest, i.e., –196°C or 77 K, therefore, it gets distilled first of all, followed by argon with boiling point –186°C or 87 K, while oxygen has the highest boiling point i.e. –183°C or 90 K, therefore, it gets distilled last of all.
The flow chart for the separation of gases of air is shown:
The actual apparatus used for separation of gases is shown in figure.
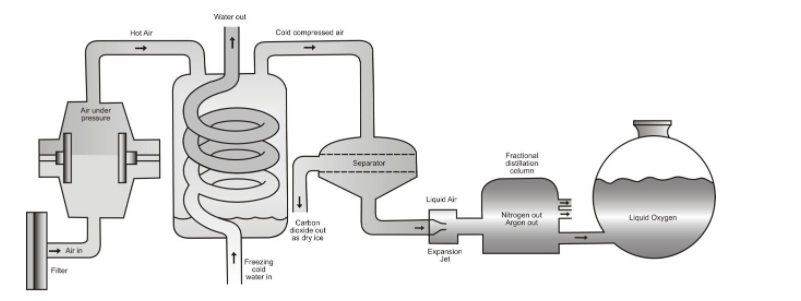
Separation of different gases from air by fractional distillation method
By Separating Funnel:
Separation of mixture of two immiscible liquids: The separation of two immiscible liquid is based on the difference in their densities. The apparatus used for separation is separating funnel. It is a long glass tube provided with a tap at its bottom. The tale bellow shows different immiscible liquids which can be separated by separating funnel.
|
Immiscible Liquid-liquid Mixture |
Heavier Liquid |
Lighter Liquid |
|
Benzene and water |
Water |
Benzene |
|
Kerosene oil and water |
Water |
Kerosene oil |
|
Turpentine oil and water |
Water |
Turpentine oil |
|
Chloroform and water |
Chloroform |
Water |
|
Mustard oil and water |
Water |
Mustard oil |
Method :
- Close the tap of separating funnel and clamp it in a vertical position in an iron stand.
- Pour the immiscible liquid mixture (say benzene-water mixture) in the separating funnel. Allow the mixture to stand for half an hour or more.
- The immiscible components of the mixture, i.e., benzene and water separate out into two distinct layers. The benzene forms the lighter layer on the top and the water forms the heavier layer at the bottom.
- Place a conical flask or a beaker under the nozzle of the separating funnel. Turn the tap gently so that the water trickles in the flask or the beaker drop by drop. Once the water is drained out, close the tap.
- Now place another conical flask or a beaker under the nozzle of separating funnel. Open the to drain out benzene.
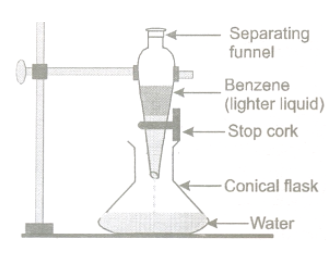
Separation by separating funnel
Applications :
- This method is used for separating any two immiscible liquids.
- This method is used in separation of stag (a waste material) form the molten metals during their extraction. For example, during the extraction of iron from its ore, the molten iron and slag collect at the base of blast furnace. The slag being less dense floats up the surface of molten iron. They are topped out from two different outlets.










