
Mechanism Of Organic Reaction
IUPAC & GOC of Class 11
A chemical equation is only a symbolic representation of chemical reaction which indicates the initial reactants and final products involved in a chemical change. Reactants generally consist of two species.
- Substrate: One which is being attacked in a chemical reaction
- Reagents: The species which attack the substrate molecule
Substrate + Reagent → Products
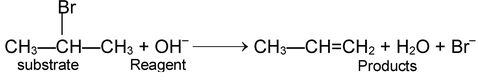
It is important to know not only what happens in a chemical reaction but also how it happens. Most of the reactions are complex and take place via reactive intermediates which may be or may not be isolated. The reaction intermediates are generally very reactive which readily react with other species present in the environment to form the products. The detailed step by step description of chemical reaction is called its mechanism. Mechanism is only a hypothesis to explain various facts regarding a chemical reaction.
Substrate → Reactive intermediates –— Products
By knowing the mechanism we can predict the product of a chemical reaction, adjust the experimental conditions to improve the yield of the products or even alter the course of reaction to get the different products.
Most of the attacking reagents carry either positive charge (an electron deficient species) or a negative charge (electron rich species). The positively charged reagents attack the substrate at points of high electron density while (-vely) charged reagents attack the point of low electron density. The organic reactions essentially involve changes in the existing covalent bonds present in the molecules. These changes may involve electronic displacements in covalent bonds breaking of some of the existing bonds (bond fission), formation of new bonds as well as energy change accompanying the bond fission and bond cleavage.
We can understand the mechanism of various organic reactions in terms of following well established basic concepts.
(i) Electronic displacement in covalent bond
(ii) Fission (cleavage) of covalent bonds
(iii) Nature of attacking reagents
Types of Organic Reactions:
All organic reactions can be broadly classified into four catagories.
(a) Substitution reactions
(b) Addition reactions
(c) Elimination reactions and
(d) Rearrangement reactions
(a) Substitution Reactions
In these an atom or a group of atoms in an organic molecule is replaced by another atom or group of atoms without any change in the remaining part of the molecule. These reactions may be initiated by free radical, electrophile or nucleophile.
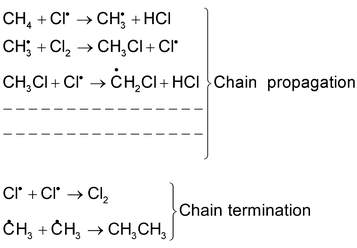
(i) Free radical substitution reaction:
This substitution reaction is brought about by free radicals. For example chlorination of methane in presence of diffused sunlight. The mechanism of the reaction is as follows.
Cl : Cl ⎯⎯→ 2Cl∙ Chain initiation
(ii) Nucleophilic substitution reoactions:
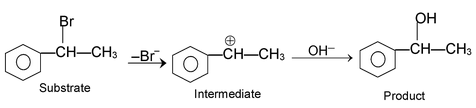
These reactions are brought about by nucleophile. The reaction can proceed either via SN1 or SN2 mechanism.
SN 1 mechanism:
Rate determining step involves only the species. For example the reaction.
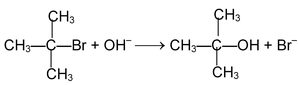
takes place as follows




