Naming Of Hydrocarbon
IUPAC & GOC of Class 11
Naming Of Hydrocarbon
Compound of Hydrogen and Carbon are called hydrocarbon
Classifications
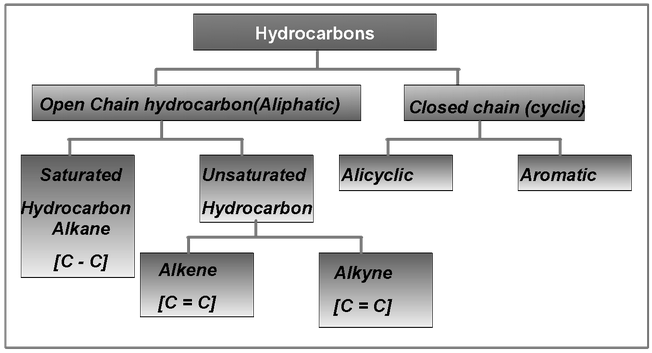
Open Chain Hydrocarbon
|
Alkane |
Alkene |
Alkyne |
|
|
General Formula |
C n H 2n+2 |
C n H 2n |
C n H 2n-2 |
|
Value of n |
C – C |
C = C |
C ≡ C |
|
n = 1 |
CH 4 (Methane) |
- |
- |
|
n = 2 |
C 2 H 6 (Ethane) |
C 2 H 4 (Ethene) |
C 2 H 2 (Ethyne) |
|
n = 3 |
C3H 8 (Propane) |
C 3 H 6 (Propene) |
C 3 H 4 (Propyne) |
|
n = 4 |
C 4 H 10 (Butane) |
C 4 H 8 (Butene) |
C 4 H 6 (Butyne) |
|
n = 5 |
C5H 12 (Pentane) |
C 5 H 10 (Pentene) |
C5H 8 (Pentyne) |
|
n = 6 |
C 6 H 14 (Haxane) |
C 6 H 12 (Hexene) |
C 6 H 10 (Hexyne) |
|
n = 7 |
C 7 H 16 (Heptane) |
C 7 H 14 (Heptene) |
C 7 H 12 (Heptyne) |
|
n = 8 |
C 8 H 18 (Octane) |
C 8 H 16 (Octene) |
C 8 H 14 (Octyne) |
|
n = 9 |
C 9 H 20 (Nonane) |
C 9 H 18 (Nonene) |
C 9 H 16 (Nonyne) |
|
n = 10 |
C 10 H 22 (Decane) |
C 10 H 20 (Decene) |
C 10 H 18 (Decyne) |
Types of carbon and hydrogen atoms in alkanes
They may be classified in to four types
(i) Primary or 10 carbon: A carbon atom attached to one other carbon atom.
(ii) Secondary or 20 carbon: A carbon atom attached to two other carbon atom.
(iii) tertiary or 30 carbon: A carbon atom attached to three other carbon atoms.
(iv) Ouaternary or 40 carbon: A carbon atom attached to four other carbons atoms.
The hydrogen atom attached to 10, 20 and 30 carbon atoms are called primary (10), secondary (20) and tertiary (30) hydrogen atoms.
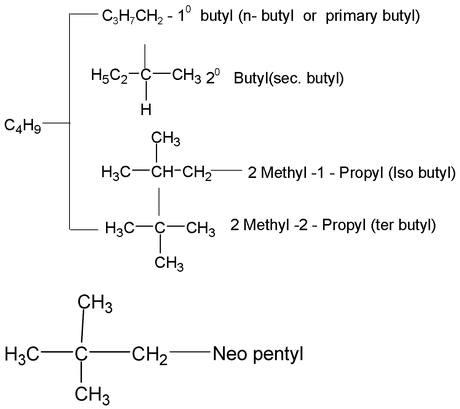
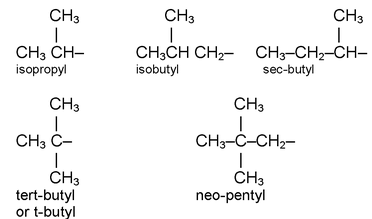
Alkyl group
The removal of one hydrogen atom from the molecule of an alkane gives an alkyl group.
General formula CnH 2n+1 n = 1, 2, 3
Example:
−CH 3 Methyl
−C 2 H 5 Ethyl
C 2 H 5 CH 2 Propyl (n – propyl) C 3 H 7
CH 3 – CHCH 3 20 Propyl (Isopropyl or secondary propyl)
Nomenclature of Hydrocarbons
Alkane
The IUPAC system of alkane nomenclature is based on the simple fundamental principle of considering all compounds to be derivatives of the longest single carbon chain present in the compound. The chain is then numbered from one end to the other, the end chosen as number 1 is that which gives the smaller number at the first point of difference.
When there are two or more identical appendages - the modifying prefixes di-, tri-, tetra-, penta-, hexa-, and so on are used, but every appendage group still gets its own number.
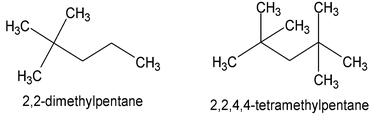
When two or more appendage locants are employed, the longest chain is numbered from the end which produces the lowest series of locants. When comparing one series of locants with another, that series is lower which contains the lower number at the first point of difference.
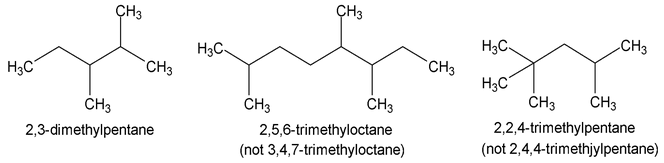
Several common groups have special names that must be memorized by the student.
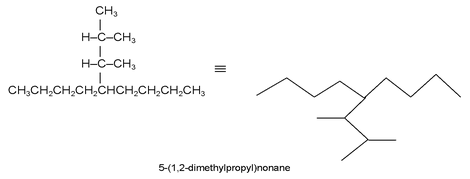
A more complex appendage group is named as a derivative of the longest carbon chain in the group starting from the carbon that is attached to the principal chain. The description of the appendage is distinguished from that of the principal chain by enclosing it in parentheses.
When two or more appendages of different nature are present, they are cited as prefixes in alphabetical order. Prefixes specifying the number of identical appendages (di, tri, tetra and so on) and hyphenated prefixes (tert-or t, sec-) are ignored in alphabetizing except when part of a complex substituent. The prefixes cyclo-, iso-, and neo-count as a part of the group name for the purposes of alphabetizing.
When chains of equal length compete for selection as the main chain for purposes of numbering, that chain is selected which has the greatest number of appendage attached to it.
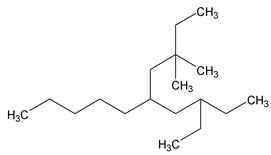
5-(2-ethylbutyl) – 3, 3 – dimethyl decane
When two or more appendages are in equivalent positions, the lower number is assigned to the one that is cited first in the name (that is one that comes first in the alphabetic listing).
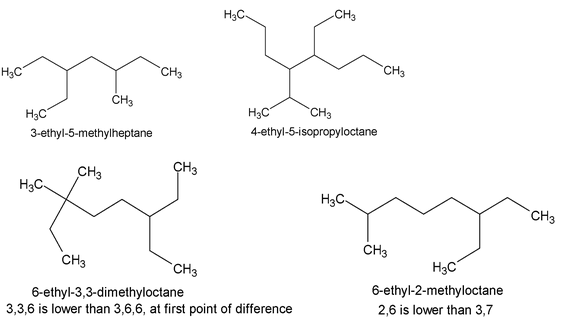
[The complete IUPAC rules actually allow a choice regarding the order in which appendage groups may be cited. One may cite the appendages alphabetically, as above, or in order of increasing complexity].
Alkenes
Nomenclature and Structure:
Alkenes (olefins) contains the structural unit
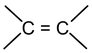
and have the general formula CnH2n. These unsaturated hydrocarbons are isomeric with the saturated cycloalkanes. The IUPAC rules for naming alkenes are similar in many respects to those for naming alkanes.
- Determine the root word by selecting the longest chain that contains the double bond and change the ending of the name of the alkane of identical length from ane to ene.
-
Number the chain so to include both carbon atoms of the double bond, and begin numbering at the end of the chain nearer the double bond. Designate the location of the double bond
- by using the number of the first atom of the double bond as prefix :
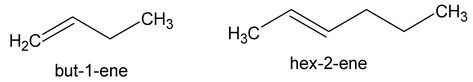
- Indicate the locations of this substituent groups by the numbers of the carbon atoms to which they are attached.
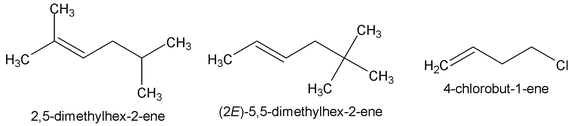
-
Two frequently encountered alkenyl groups are the vinyl group and the allyl group.
CH 2 = CH — CH 2 = CH CH 2 —
The vinyl group The allyl group (are not included in IUPAC system)
The following examples illustrate how these names are employed
CH 2 = CH — Br CH 2 = CH — CH 2 Cl
vinyl bromide allyl chloride (are not IUPAC name)
- The geometry of the double bond of a disubstituted alkene is designated with the prefixes, cis and trans. If two identical group are on the same side of the double bond, it is cis, if they are on opposite sides; it is trans.
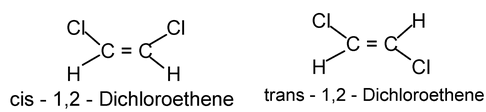
Alkyne
Nomenclature of Alkynes:
Alkynes contains the structural unit

and have the general formula CnH 2n-2 .
The simple alkynes are readily named in the common system as derivatives of acetylene itself.
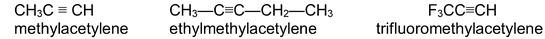
In the IUPAC system the compounds are named as alkynes in which the final – ane of the parent alkane is replaced by the suffix – yne. The position of the triple bond is indicated by a number when necessary.
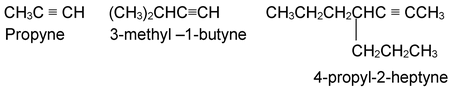
When both a double and triple bond are present, the hydrocarbon is named an alkenyne with numbers as low as possible given to the multiple bonds. In case of a choice, the double bond gets the lower number.
In complex structures the alkynyl group is used as a modifying prefix.
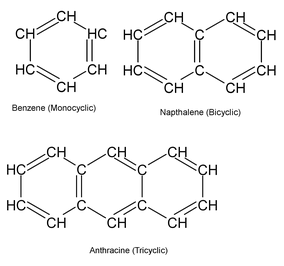
Aromatic Hydrocarbons
These compounds consists of at least one benzene ring, i.e., a six – membered carbocylic ring having alternate single and double bonds.