
Reaction Intermediates
IUPAC & GOC of Class 11
Reaction Intermediates
Most of organic reactions occurs through the involvement of certain chemical species. These are generally short lived (10-6 seconds to a few seconds) and highly reactive and hence can not be isolated. These short lived highly reactive chemical species. Through which the majority of the organic reactions occur are called reactive intermediates. These intermediates are detected by spectroscopic methods or trapped chemically or their presence is confirmed by indirect evidence. On the other hand, synthetic intermediate are stable products which are prepared isolated and purified and subsequently used as starting materials in a synthetic sequence.
Carbocations (Earlier Called As Carbonium Ions)
Carbocations are the key intermediates in several reactions and particularly in nucleophilic substitution reactions and electrophilic addition reaction.
(a) Structure:
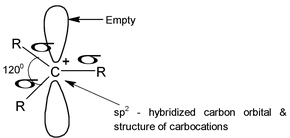
Generally in the carbocations the positively charged carbon atom is bonded to three others atoms and has no nonbonding electrons. It is sp2 hybridized with a planer structure and bond angles are of about 1200. There is a vacant unhybridised p orbital which (e.g in the case of
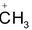 lies perpendicular to the plane of C ⎯ H bonds.
lies perpendicular to the plane of C ⎯ H bonds.
(b) Stability:
There is an increase in carbocation stability with additional alkyl substitution. Thus one finds that addition of HX to three typical olefins decreases in the order
(CH
3
)
2
C = CH
2
> CH
3
⎯ CH = CH
2
> CH
2
= CH
2
This is due to the relative stabilities of the carbocations formed in the rate determining step which in turn follows from the fact that the stability is increased by the electron releasing methyl group (+I), three such groups being more effective than two, and two more effective than one.
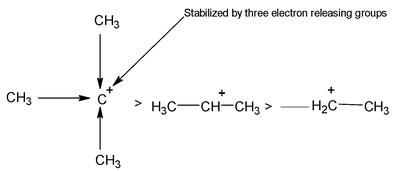
Stability of carbocations 30 > 20 > 10 >
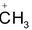
Electron release: Disperses charge, stabilizasion.
Further, any structural feature which tends to reduce the electron deficiency at the tricoordinate carbon stabilizes the carbocation. Thus when the positive carbon is in conjugation with a double bond. The stability is more. This is so, due to resonance the positive charge is spread over two atoms instead of being concentrated only on one. This explains the stability associated with the allylic cations. The benzylic cations are stable, since one can draw canonical forms as for allylic.




