Metallurgy
Metal And Non-metals of Class 10
METALLURGY
Extraction of metals from below the surface of the earth forms a major industrial endeavor. Since most metals are reactive, most of them are not found as free elements under the earth’s surface. Relatively unreactive metals like silver (Ag), gold (Au), Platinum (Pt) are found as pure elements. Copper (Cu) is found in free state as well as in combined state. Naturally occurring materials or compounds which are a combination of metals with other elements are called minerals. Minerals are found amongst rocks and stones inside the earth’s crust. Such rocks and stones are called mineral ores. Ores have to be mined, crushed, and metals have to be extracted from them by various chemical processes. These processes form a separate branch of engineering called metallurgy. Thus metallurgy deals with extraction and refining or ores.
The earth’s crust is the major source of metals. Sea water also contains some soluble salts of metals like sodium chloride, magnesium chloride etc.
Some metals are found in the earth’s crust in free state where as some are found in the form of their compounds. The metals at the bottom of activity series are least reactive. Therefore, they are often found in free state. For e.g. gold, silver, platinum, copper.
The metals at the top of activity series (K, Na, Ca, Mg etc.) are so reactive, that they are never found in nature as free elements but are found in the form of their compounds. The metals in the middle of activity series (Al, Zn, Fe, Pb etc.) are moderately reactive. They are found in earth’s crust mainly as oxides, sulphides or carbonates.
Thus, the metals occur in nature in two forms :
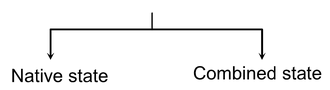
(a)Native State : A metal is said to exist in native state if it exists in the elementary form in the crust of the earth.
(b)Combined State : A metal is said to exist in combined state if it is found in the earth’s crust in the form of its stable compounds.
MINERALS AND ORES
In the combined state, the metals are found in the crust of earth as oxides, carbonates, sulphides, silicates, phosphates etc.
The inorganic elements or compounds which occur naturally in the earth’s crust are known as minerals. At some places, the minerals contain a very high percentage of a particular metal and the metal can be profitably extracted from it. Those minerals from which a metal can be profitably extracted are called ores.
When an ore is mined from earth, it is always found to be contaminated with sand and rocky materials. These impurities of sand and rocky materials present in the ore are known as gangue.
TYPES OF ORES
Most of the ores are either oxides or sulphides. Some of the ores are also carbonates and halides. The various types of ores and their examples are given below in the table :
Various Types of Ores
|
S.No. |
Type |
Examples |
|
1. |
Oxide ores |
Haematite (Fe 2 O 3 ) Bauxite (Al 2 O 3 . 2H 2 O) Magnetite (Fe 3 O 4 ) |
|
2 |
Sulphide ores |
Copper pyrite (CuFeS 2 ) Iron pyrite (FeS 2 ) Zinc blende (ZnS) Cinnabar (HgS) |
|
3. |
Carbonate ores |
Limestone (CaCO 3 ) Siderite (FeCO 3 ) Calamine (ZnCO 3 ) |
|
4. |
Halide ores |
Rock salt (NaCl) Horn silver (AgCl) Fluorspar (CaF 2 ) |
Some common ores are given in table below :
Some Common Ores
|
S.No. |
Elements |
Ores |
|
1. |
Iron |
Haematite (Fe 2 O 3 ) Magnetite (FeO . Fe 2 O 3 ) Iron pyrites (FeS 2 ) |
|
2 |
Aluminium |
Bauxite (Al 2 O 3 . 2H 2 O) |
|
3. |
Manganese |
Pyrolusite (MnO 2 ) |
|
4. |
Calcium |
Limestone (CaCO 3 ) |
|
5. |
Limestone |
Dolomite (MgCO 3 . CaCO 3 ) |
|
6. |
Copper |
Copper pyrites (CuFeS 2 ) |
|
7. |
Mercury |
Cinnabar (HgS) |
|
8. |
Zinc |
Zinc blende (ZnS), Calamine (ZnCO 3 ) |
|
9. |
Lead |
Lead glance (PbS) |
|
10. |
Sodium |
Rock salt (NaCl) |
|
11. |
Silver |
Horn silver (AgCl) |
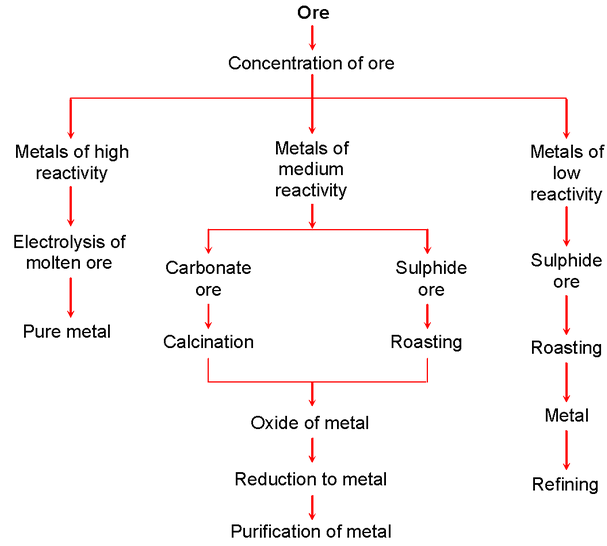
These ores have to be extracted and converted into free metals, purified and refined for their use as metals for industrial applications. The various steps taken in metallurgy for obtaining metals in pure form are :
1. Concentration of ore : Ore is purified and concentrated. Unwanted rocks and grit are eliminated in this process. Hydraulic washing, froth floatation process, magnetic separation and chemical separation are some of the techniques used for concentrating or purification of ores.
2. Conversion into metal oxide : After an ore is removed of all unwanted rocks and grit, the ore is chemically converted into an oxide. This is because it is easy to reduce a metal-oxide to metal by various known processes. A carbonate ore is made into a metal-oxide by calcination. A sulphide ore is converted into a metal-oxide by heating the ore in air.
3. Reduction of metal-oxide : Metal oxides are reduced by processes such as electrolytic reduction. Metal is obtained, but this may not be pure metal as there may be mixtures of metals
4. Refining of impure metal : In a given ore, there may be more than one metal. To obtain a certain metal, the combination of metals obtained from reducing processes has to be refined. The refining and purification process eliminates unwanted metal impurities. There are many methods of purification. They are electrolysis, distillation, chemical methods, etc.
Metals that are very reactive, such as sodium (Na) and potassium (K), are extracted by electrolysis of chlorides. Calcium in the form of fluorspar (CaF2) is also extracted by electrolysis. Al is sometimes extracted from bauxite by electrolysis.
Concentration of ore
Unwanted rocks, sand and grit from the mineral ore are called gangue or matrix. These have to be removed so that the mineral ore is concentrated with higher percentage of metal. Ores are mined from deep within the earth’s crust in the form of rocks. The minerals are embedded in these rocks. The rocks are first crushed into smaller pieces by crushers. Then they are ground to powder by ball mill and other processes so that powdered ore is obtained. Depending on the type of ore, hydraulic washing, froth floatation process, magnetic separation and chemical separation techniques are applied for concentrating an ore.
Hydraulic washing
It is used for the enrichment of oxide ores. It is based on the difference in the densities of the gangue and the ore particles. The gangue particles are generally lighter as compared to ore particles. In this process, the crushed and finely powdered ore is washed with a stream of water. The lighter gangue particles are washed away, leaving behind the heavier ore particles.

Hydraulic Washing
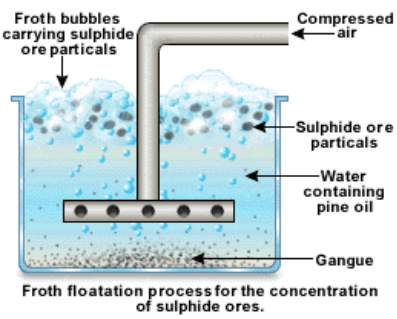
Froth Floatation Process
It is used to separate the gangue from the sulphide ores, especially those of copper, zinc and lead. In this process, the finely powdered ore is mixed with water in a large tank to form a slurry. Then some pine oil is added to it. The sulphide ores are preferentially wetted by the pine oil, whereas the gangue particles are wetted by the water. When air is blown through the mixture, the lighter oil froth carrying the metal sulphides rises to the top of the tank and floats as scum. It is then skimmed off and dried. The gangue particles being heavier, sink to the bottom of the tank.
Electromagnetic Separation
It is used to enrich the magnetic ores (iron ore). The separation is done by using magnetic separators. A magnetic separator consists of a leather belt that moves over two rollers, out of which one is an electromagnet. The finely powdered ore is dropped over the moving belt at one end. When the ore falls down from the moving belt at the other end, the magnetic portion in the ore is attracted by the magnet and forms a heap nearer to the roller. The non-magnetic impurities fall away from the magnetic ore and form a separate heap.
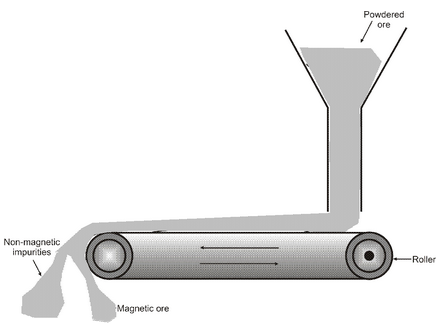
Magnetic separation : The finely powdered ore is dropped over a moving belt which passes over a magnetic roller. The particles attracted by the magnet form a separate pile.
Chemical Separation
The process of chemical separation makes use of differences between the chemical properties of the gangue and the ore.
For example, bauxite, Al 2 O 3 . 2H 2 O, is an impure form of aluminium oxide. The impurities mainly present in it are iron (III) oxide (Fe 2 O 3 ) and sand (SiO 2 ). The iron (III) oxide gives it a brown-red colour. Baeyer’s method is used to obtain pure aluminium oxide from bauxite ore. In this method, the finely powdered ore is treated with hot sodium hydroxide solution. The aluminium oxide present in bauxite ore reacts with sodium hydroxide to form water soluble sodium aluminate.
Al 2 O 3( s) + 2NaOH (aq) → 2NaAlO 2 (aq) + H 2 O(l)
(From impure bauxite ore) Sodium aluminate
Iron (III) oxide does not dissolve in sodium hydroxide solution. It is, thus, separated by filtration. Silica reacts with sodium hydroxide to form water soluble sodium silicate.
SiO 2 + 2NaOH → Na2 SiO 3 + H 2 O
Sodium silicate
The filtrate containing sodium aluminate and sodium silicate is then stirred with some aluminium hydroxide to induce the precipitation of aluminium hydroxide. The impurity, silica, remains dissolved as sodium silicate in the solution.
Na AlO 2 (aq) + 2H 2 O (l) → Al(OH) 3 (s) + NaOH(aq)
Aluminium hydroxide
Aluminium hydroxide is then filtered off, washed, dried and ignited to get pure aluminium oxide, which is called alumina.
Alumina
Conversion of ore into metal oxide
Ores concentrated by any of the above processes are still in their original oxide, carbonate, sulphide or halide forms. To obtain pure metals from these chemicals, it is better convert the metal-compounds into oxides. Oxides of metals can be reduced easily and pure metal can be obtained. To convert the concentrated ores into metal oxides, two processes are used. These processes are calcination (heating in inadequate quantity of air ) and roasting the ore (heating in adequate quantity of air).
-
Calcination : Carbonate ores are heated in absence of air. The absence of air and heat converts the CO3 into CO2 and O. The O remains with the metal as metal-oxide. Heating also expels any water content in the ore. In case these are any volatile impurities or gases trapped in the ore, they are also removed by heating.
-
Roasting : Sulphide ores are roasted or heated in plenty of air. The sulphide S changes to sulphur dioxide. The metal reacts with oxygen in the air to become a metal-oxide. Heating removes gaseous and other volatile impurities.
Reduction of metal-oxide
Reduction process is used for converting metal-oxides into metal. Metal-chloride can also be reduced directly. The reduction reaction chosen depends on the chemical reaction or reactivity of metals. The method used for the extraction of the metal from the concentrated ore depends upon the nature of the metal. Based on their reactivity, the metals have been grouped into three categories :
(a) Metals of low reactivity
(b) Metals of medium reactivity
(c) Metals of high reactivity
The activity series and related metallurgy is given below :
|
Metal |
Method of extraction |
|
K Na Ca Mg Al |
Electrolysis of molten chloride or oxide |
|
Zn Fe Pb Cu |
Reduction of oxide with carbon |
|
Cu Hg |
Heating sulphide in air (Reduction by heat alone) |
|
Ag Au Pt |
Found in native state (as metals) |
Extraction of metals low in the Activity Series
Metals low in the activity series are very unreactive. The oxides of these metals can be reduced to metals by heating alone. For example
(a) Cinnabar (HgS) is an ore of mercury. When it is heated in air, it is first converted into mercuric oxide (HgO).
2HgS(s) + 3O 2 (g) → 2HgO(s) + 2SO 2 (g)
Mercuric sulphide Oxygen Mercuric oxideSulphur dioxide
Mercuric oxide is then reduced to mercury on further heating.
2HgO(s)
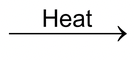 2Hg(l) + O2(g)
2Hg(l) + O2(g)
Mercuric oxide Mercury metal Oxygen
(b) Similarly, when copper glance (Cu 2 S), an ore of copper, is subjected to roasting, it directly gives copper according to the following reactions :
2Cu
2
S(s) + 3O
2
(g)
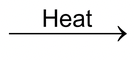 2 Cu
2
O (s) + 2 SO
2
(g)
2 Cu
2
O (s) + 2 SO
2
(g)
Copper glance Oxygen (from air) Cuprous oxide Sulphur dioxide
or Copper (I) oxide
2Cu
2
O(s) + Cu
2
S(s)
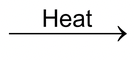 6 Cu (s) + 2 SO
2
(g)
6 Cu (s) + 2 SO
2
(g)
Cuprous oxide Copper glance Copper Sulphur dioxide
Extraction of Metals in the Middle of Activity Series
The metals in the middle of the reactivity series such as iron, zinc, lead, copper etc. are moderately reactive.
These metals are found in nature in the form of their oxide, sulphide or carbonate ores. Further, as it is easier to reduce oxides than sulphides or carbonates, therefore, the sulphide and carbonate ores are first converted into the corresponding metal oxides. Thus, the different steps involved for the extraction of the metal from the concentrated ore are as follows :
(a)Conversion of the carbonate or sulphide ore into metal oxide. This is done by either of the following two methods :
(i) Calcination (For carbonate ores). It is the process of heating the ore strongly in the absence of air. The metal carbonate decomposes to form metal oxide. For example,
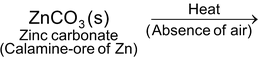 ZnO(s) + CO
2
(g)
ZnO(s) + CO
2
(g)
(ii)Roasting (For sulphide ores). It is the process of heating the ore strongly in the presence of excess of air. As a result, the sulphide ore is converted into metal oxide. For example,

(b)Reduction of the metal oxide to metal. As these metals are moderately reactive, their oxides cannot be reduced by heating alone. Hence, their oxides are reduced to metals by using a suitable reducing agent such as carbon or some highly reactive metals like sodium, calcium, aluminium etc. A few examples are :
(i)Reduction by heating with carbon. Carbon in the form of coke or coal is the cheapest reducing agent. It combines with the oxygen of the metal oxide forming carbon monoxide. As a result, metal oxide is reduced to metal. For example, oxides of iron and zinc are reduced to their respective metals by heating with coke.
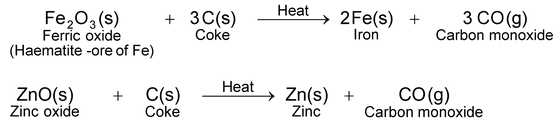
Carbon monoxide formed also acts as reducing agent and further reduces the metal oxide to metal.
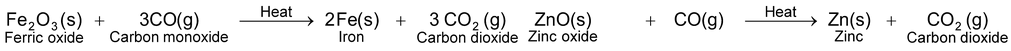
(ii)Reduction by heating with aluminium. Oxides of certain metals, e.g., manganese oxide (MnO 2 ), chromium oxide (Cr 2 O 3 ), etc. cannot be satisfactorily reduced by heating with carbon. However, these oxides are easily reduced to their corresponding metals by heating with aluminium powder (as aluminium is more reactive than manganese or chromium). As a result, aluminium is converted into aluminium oxide whereas the metal oxide is reduced to the metal. The reaction is highly exothermic. The heat evolved is so high that the metal is obtained in the molten state.
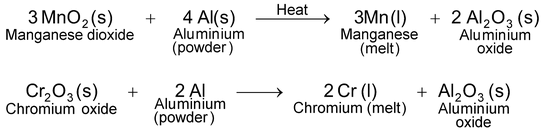
Similarly, when iron (III) oxide is heated with aluminium powder (the mixture is ignited by inserting a magnesium ribbon and then burning it), the heat evolved is so high that iron obtained melts.

This reaction is, therefore, used for welding the broken parts of iron machinery, railway tracks etc. The reaction is known as thermit reaction.
The reduction of metal oxides to metal using aluminium as the reducing agent is called aluminothermy.
Extraction of Metals towards the Top of Activity Series
The metals high up in the reactivity series are very reactive. They cannot be obtained from their compounds by heating with carbon. For example, carbon cannot reduce the oxides of sodium, magnesium, calcium, aluminium, etc., to the respective metals. This is because these metals have more affinity for oxygen than carbon. These metals are obtained by electrolytic reduction. For example, sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
During electrolysis, the metal ions, being positive are liberated at the cathode (the negatively charged electrode), whereas the chlorine is liberated at the anode (the positively charged electrode). So, during the electrolysis of molten salts, the metals are always produced at the cathode (negative electrode).
For example :
(i)Electrolysis of molten sodium chloride. When sodium chloride melts, it splits into sodium ion (Na + ) and chloride ion (Cl – ).
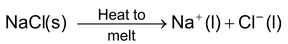
When electricity is passed through the melt, Na + ions go to the cathode whereas Cl – ions are liberated at the anode. Na + ions gain electrons at the cathode and are thus reduced to sodium atoms. Cl – ions lose electrons at the anode and are thus oxidized to chlorine atoms. These chlorine atoms then combine with each other to form chlorine (Cl 2 ) gas. The reactions taking place are as follows :
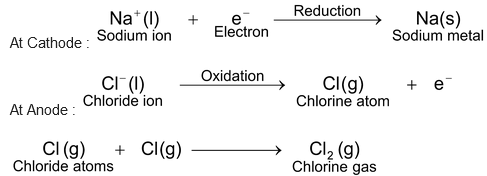
Thus, sodium metal is obtained at the cathode whereas chlorine gas is liberated at the anode.
Similarly, calcium and magnesium are also obtained by the electrolysis of their fused chlorides.
(ii)Electrolysis of molten alumina. Alumina (Al 2 O 3 ) is a stable compound. Hence, it cannot be reduced to aluminium by heating with carbon. It is obtained by electrolytic reduction of the molten alumina. Molten alumina (Al 2 O 3 ) contains aluminium ions (Al 3+ ) and oxide ions (O 2– ). On passing electricity, the reactions taking place at the electrodes are as follows :
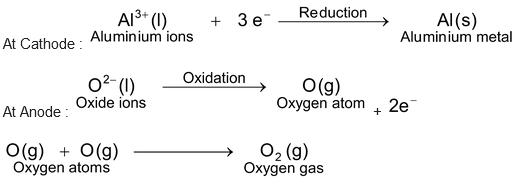
Refining of metals
Free metals obtained by various reduction processes may have several impurities which may be other metals. For example while obtaining Na, K is a common metal that may be separated along with Na. Thus K is an impurity here. These impurities have to be removed by processes known as refining. Refining is nothing but purification of metals. There are various methods of refining; some of which we will discuss below.
(i)Liquation method : In liquation method, metals with low melting points are refined. Metals like Sn, Pb, Bi have low melting points compared to the impurities in the metal. A block of impure metal is placed on a sloping furnace. The temperature of the furnace is maintained a bit above the melting point of the metal to be refined. The metal melts and flows off the slope, it is then collected and cooled.
(ii)Distillation method : Zn, Cd and Hg form vapours easily. They can be heated and distilled out from their impurities. Impure metal block is heated at a temperature so that the metal atoms start to evaporate. The temperature is then held constant. The vapours are condensed and separated out in a container called receiver. Non-volatile impurities are left behind in the distillation chamber.
(iii)Oxidation method : Sometimes impurities are able to get oxidized more easily than the metal itself. In this case oxidative method is used. For example if impurities are S, C, Si or P, they can get oxidized more easily than the metal itself. For example in case of pig iron Fe, these non-metals are present as impurities. When air is passed over hot molten pig iron, these non-metals get oxidized to CO 2 , SO 2 , P 2 O 5 and can be removed easily.
(iv)Electrolytic refining : In this method electrolysis is used to refine metals. Metals like Cu, Zn, Sn, Pb, Ag, Au are refined by electrolysis method. In an electrolytic cell, a block of impure metal is made into the anode, a thin strip of pure metal is made into a cathode, and an electrolyte is made out of a suitable metal-salt of the metal to be refined. When an electric current is passed through the cell, ions from the anode enter the electrolyte. The same number of metal ions from the electrolyte gets deposited on the cathode. This is a preferential deposition. Impurities remain in the electrolyte. Some of the impurities may be deposited below the anode. As an example we will study electrolytic refining of copper,
Electrolytic refining of copper: In an electrolytic tank, acidified copper sulphate (CuSO 4 + dil H 2 SO 4 ) solution forms the electrolyte. A block of impure copper is made into an anode by connecting the positive terminal of a power supply (battery). A thin strip of highly pure copper metal is the cathode of the cell. The negative terminal of the power supply is connected to it.
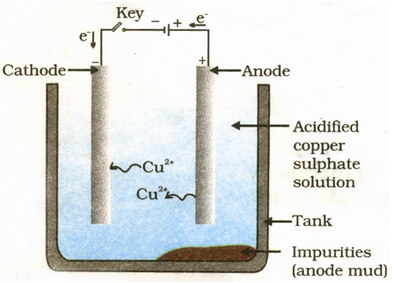
A small electric current is passed through the cell. Atoms from the anode enter the electrolyte. The copper from the anode gets converted into copper sulphide. An equal number of copper atoms from the solution get deposited on the cathode. This is to keep the concentration of the solution constant. Impurities from the anode block either remain in solution or collect below the anode, as they are unable to displace copper form the sulphate solution. The impurities remain insoluble in the electrolyte and they are called anode mud.
Copper sulphate solution contains ions of Cu
++
and SO
4
—
.The following reactions take place at the anode and cathode when an electric current is passed.
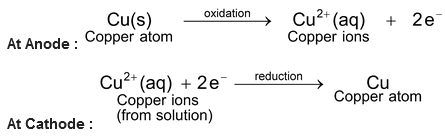
Pure copper is scraped or removed from the cathode. Anode becomes thinner as the electrolysis process proceeds. Some important metals like gold and silver are present in the anode mud. These can be recovered separately.