
Classification of elements
Metal and Non-metals of Class 8
CLASSIFICATION OF ELEMENTS INTRODUCTION
It was found that there was a wide variation in the properties of elements. Hence these were further classified into three categories, i.e. metals, non-metals, metalloids based on the properties they exhibit.
Elements can be classified as a metal, nonmetal, or metalloid.
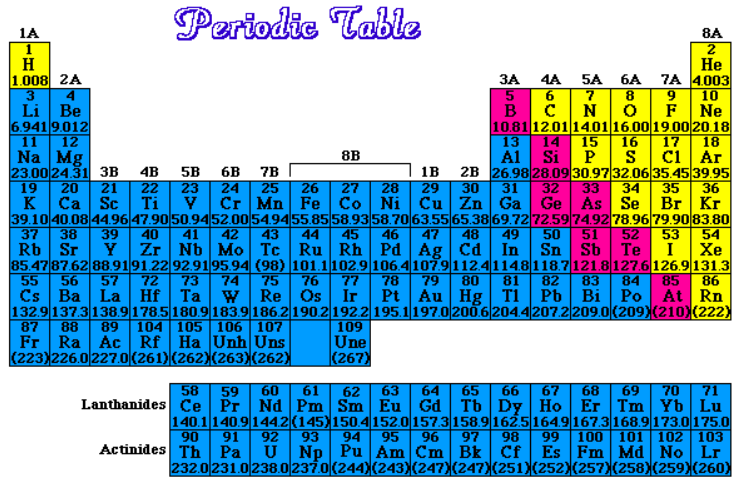
The elements of the periodic table were placed into various divisions based on their chemical and physical properties.
METALS:
Metals are located to the left of the "staircase" on the periodic table and are shown in blue in the table above.
Their properties include:
- Malleable
- Ductile
- Lustrous
- Good conductors of heat and electricity
- Solid at room temperature (except Hg)
NON METALS:
Nonmetals are located to the right of the "staircase" on the periodic table and are shown in yellow on the table above.
Their properties include:
- Not generally solids at room temperature. Most are gasses, some are solids, Br is a liquid.
- Not malleable
- Not Ductile
- Low luster
- Bad conductors of heat and electricity
METALLOIDS:
Metalloids are located along the "staircase" on the periodic table and are shown in pink on the table above.
Their properties include those of both metals and nonmetals. For example, Germanium is lustrous like a metal, but not malleable like a nonmetal.
Another way an element can be a metalloid is if under some conditions it behaves as a metal, but under other conditions the same metalloid behaves as a nonmetal. For example, at room temperatures Boron is a poor electrical conductor (like nonmetals) but is a good conductor (like metals) at high temperatures.
Some common metalloids are arsenic, antimony and silicon. The noble (inert) gases from the fourth category of elements.









