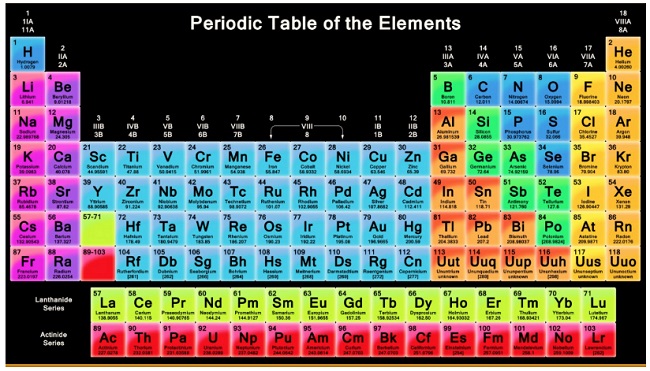
Modern Periodic Table
Periodic Classification of Class 10
In 1913, an English physicist, Henry Moseley showed that the physical and chemical properties of the atoms of the elements are determined by their atomic number and not by their atomic masses. Consequently, the periodic law was modified.
MODERN PERIODIC LAW (MOSELEY'S PERIODIC LAW):
“Physical and chemical properties of an element are the periodic function of its atomic number”. The atomic number gives us the number of protons in the nucleus of an atom and this number increases by one in going from one element to the next. Elements, when arranged in the order of increasing atomic number Z, lead us to the classification known as the Modern Periodic Table. Prediction of properties of elements could be made with more precision when elements were arranged on the basis of increasing atomic number.
PERIODICITY:
The repetition of elements with similar properties after certain regular intervals, when the elements are arranged in order of increasing atomic number, is called periodicity.
CAUSE OF PERIODICITY:
The periodic repetition of the properties of the elements is due to the recurrence of similar valence shell (outermost shell) electronic configuration after certain regular intervals.
e.g., Alkali metals have similar electronic configuration (ns 1 ) and therefore have similar properties.
ALKALI METALS
|
At. N. |
Element |
Symbol |
Electronic Configuration |
|
3 |
Lithium |
Li |
2,1 |
|
11 |
Sodium |
Na |
2,8,1 |
|
19 |
Potassium |
K |
2,8,8,1 |
|
37 |
Rubidium |
Rb |
2,8,18,8,1 |
|
55 |
Caesium |
Cs |
2,8,18,18,8,1 |
|
87 |
Francium |
Fr |
2,8,18,32,18,8,1 |
LONG FORM OF PERIODIC TABLE:
- The long form of periodic table is based upon Modern periodic law. Long form of periodic table is the contribution of Range, Werner, Bohr and Bury.
- This table is also referred to as Bohr's table since it follows Bohr's scheme of the arrangement of elements into four types based on electronic configuration of elements.
- Long form of periodic table consists of horizontal rows (periods) and vertical columns (groups).
CHARACTERISTICS OF PERIODS
- First period is called shortest period and contains only two elements. Second and third periods are called short periods containing eight elements each. Fourth and fifth periods are long periods containing eighteen elements each. Sixth period is longest period with thirty-two elements. Seventh period is an incomplete period containing nineteen elements. Numbers 2, 8, 18, 32 are called magic numbers.
- Lanthanide and actinide series containing 14 elements each are placed separately under the main periodic table. These are related to sixth and seventh periods of III group respectively.
- Elements of third period from sodium (Na) to Chlorine (Cl) are called representative or typical elements.
- Valency of an element in a period increases from 1 to 7 with respect to oxygen.
Na 2 O MgO Al 2 O 3 SiO 2 P 2 O 5 SO 3 Cl 2 O 7
1 2 3 4 5 6 7
- From left to right in a period generally
- Atomic weight, effective nuclear charge, ionisation potential, electronegativity and electron affinity of an element increases.
- Atomic radius, electropositive character and metallic character of an element decreases.
CHARACTERISTICS OF GROUPS
- Mendeleev’s periodic table contains nine groups. These are represented by Roman numerals I, II, III, IV, V, VI, VII, VIII and zero,. Groups I to VII are divided into two subgroups A and B, Group VIII consists of three sets, each one containing three elements.
- Inert gases are present in zero group. These were not discovered till that time.
- The valency of an element in a group is equal to the group number.
- There is no resemblance in the elements of subgroups A and B of same group, except valency
- The elements of the groups which resemble with typical elements are called normal elements. For example IA, IIA, IIIA, IVA, VA, VIA, VIIA group elements are normal elements
- Those elements of the groups which do not resemble with typical elments are called transition elements. For example- IB, IIB, IIIB, IVB, VB, VIB, VIIB, and VIII group elements are transition elements.
- Hydrogen can be placed in both IA and VIIA groups.
- In a group, from top to bottom in general
- Atomic weight, atomic size, electropositive character and metallic character of an element increases.
- Ionisation potential, electron affinity and electronegativity of an element decreases
MERITS OF LONG FORM OF PERIODIC TABLE:
- The long form of periodic table is based on atomic number. Atomic number is a more fundamental property of an element as compared to atomic mass.
- In the long form of periodic table, different isotopes can be placed at the same place because they have same atomic number. On the other hand, isobars such as Ar (40) and Ca (40) have to be placed at different places due to their different atomic numbers.
- The long form of periodic table can explain why all the elements in a group have similar properties while the elements in a period have different properties:-
The basis for periodicity of elements is the similar electronic configuration of the outermost shell of elements of the same group. The similar electronic configuration of the elements is repeated at regular intervals so the properties of the elements are also repeated at regular intervals.
- It is easy to remember and reproduce the table.
LIMITATIONS OF LONG FORM OF PERIODIC TABLE:
- Position of hydrogen is not accurate.
- Inner transition elements (Lanthanoids and Actinoids) have been given separate positions below in the periodic table.