
Ester
Nov 09, 2022, 16:45 IST
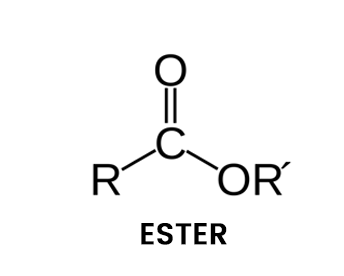
An ester is a chemical compound which is derived from an acid (inorganic or organic) in which at least 1 hydroxyl group –OH is replaced by an –O–alkyl (alkoxy) group. In general terms, esters are a group of chemical compounds formed by binding an alcohol group with a group of organic acids, the loss of water molecules.
| Table of Content |
Esters are usually derived from the carboxylic acids. It can also be obtained by reacting acid anhydride or acid halides with alcohols or carboxylic acid salts with alkyl halides.
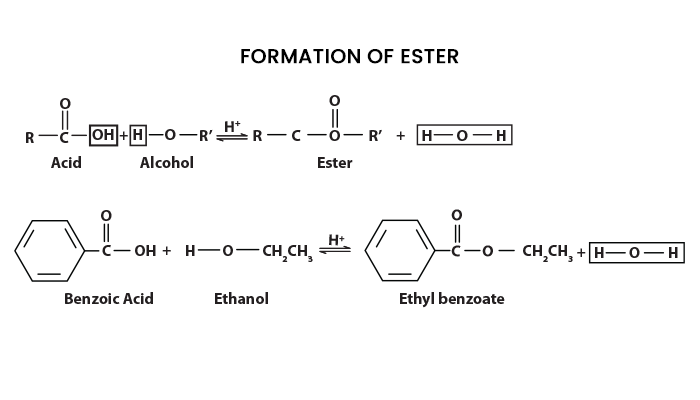
What is Esterification?
Esterification is a process or general name for a chemical reaction in which two reactants (an acid and an alcohol) form an ester as a reaction product. The formula for carboxylic acid esters is RCOOR' (where R and R' are any organic combining groups), which are prepared by reacting alcohols and carboxylic acids in the presence of hydrochloric acid or sulfuric acid as a catalyst.
We can talk about ethyl ethanoate to give you an example of an ester. Here, the hydrogen in the -COOH group is usually replaced by an ethyl group. The formula for ethyl ethanoate can be found below:
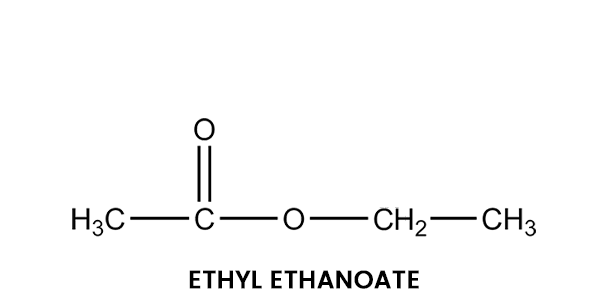
In this case, the ester is named oppositely from how the formula is written. In this case, the “ethanoate” part comes from ethanoic acid, and the “ethyl” part comes from the ethyl group at the end.
Structure of Ester
Esters are characterized by a carbon-oxygen double bond that is also single-bonded to the second oxygen atom. The oxygen atom is further attached to an aryl or alkyl group. They come in all shapes and sizes. They can take a form as small as allyl hexanoate, like pineapple odor, or as large as a long-chain triglyceride like soybean oil.
Ethyl Acetate
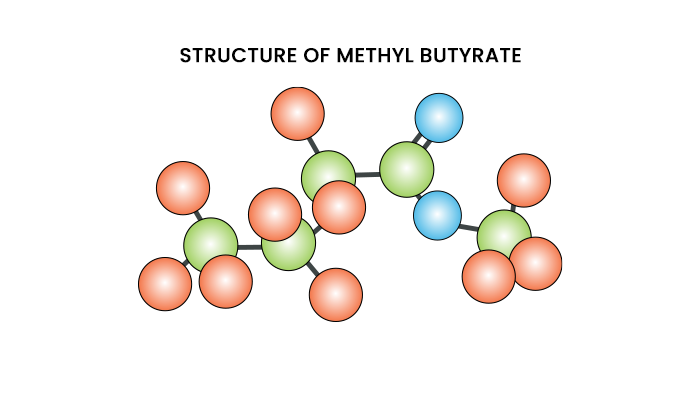
Methyl Butyrate
Generally, esters can split back to the alcohols and the carboxylic acids by the action of dilute acid, water or dilute alkali. This process is known as ester hydrolysis.
Uses of Esters
Generally, It is a sweet-smelling substance. Some of these are used as food flavors, and also other esters are used as fragrances or perfumes. In addition, they can be converted into polymers called polyesters, that can be used to make cans or plastic bottles.
Some other users of esters-
- Esters have a fragrant odor and are used as a constituent in perfumes, essential oils, food flavors, cosmetics, etc.
- It is used as organic solvent.
- Natural esters are found in pheromones.
- Naturally occurring oils and fats are esters of glycerol and fatty acids.
- Nitrate esters such as nitroglycerin is used as explosive materials.
- Polyesters can be further converted into fibers for the manufacture of clothing.
- Surface-active substances such as soap and detergents are made from it.
Frequently Asked Questions (FAQs)
Q1. Why are esters volatile?
Ans. Because of their lack of the hydrogen bond-donating ability, esters do not self-associate. Generally, esters are more volatile than the carboxylic acids of similar molecular weight.
Q2. Is ester polar or nonpolar?
Ans. Polar molecules
Q3. Are esters acidic or basic?
Ans. Esters are neutral compounds, the acids from which they are formed. In the complex reactions, the alkoxy (OR′) group of an ester is replaced by the another group. Such reaction is hydrolysis, literally “splitting with water.” Both acid and a base catalyze the hydrolysis of esters.
Q4. Are esters flammable?
Ans. Many esters are both flammable or highly flammable. Low-molecular-weight esters like methyl formate have low flash points and also wide flammability limits, making them dangerous flammability hazards.
Q5. Do esters need heat to form?
Ans. Esters are produced when the carboxylic acids are heated with the alcohols in the presence of an acid catalyst.









