
The Dichloroacetic Acid Formula, or DCA, is an organochlorine compound that shares similarities with acetic acid. It differs from acetic acid by having two chlorines replace two hydrogen atoms in the methyl group. Other names for this formula include bichloracetic acid and dichloroacetate. Its property values include a hydrogen bond donor value of 1 and a hydrogen bond acceptor value of 2, as well as a rotatable bond count of 1. This formula is the acidic counterpart to acetic acid and has many uses, similar to other chloroacetic acids. Additionally, its esters and salts are known as dichloroacetates. Scientists have shown interest in using these salts as potential therapeutics due to their ability to inhibit the pyruvate dehydrogenase kinase enzyme.
Dichloroacetic Acid Formula and Structure
The chemical compound of dichloroacetic acid is CHCl 2 COOH and its molecular formula is C 2 H 2 CL 2 O 2 . As an organochlorine compound of acetic acid, it has two of the three hydrogen atoms of the methyl group substituted by chlorine atoms in its molar mass of 128.94 g/mol.
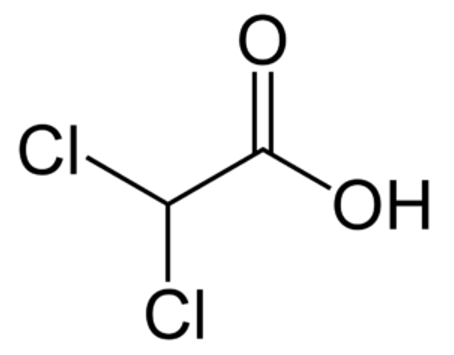
Occurrence of Dichloroacetic Acid
We can derive it from acetic acid and it is the conjugate acid of a dichloroacetate. Furthermore, it is a monocarboxylic acid and an organochlorine compound.
Also Check – Vapor Pressure Formula
Properties of Dichloroacetic Acid
In addition, it mixes easily with water and is corrosive to metal and tissues. Its density is 1.56 grams per cubic centimeter.
| Molecular formula | CHCl 2 COOH (or) C 2 H 2 CL 2 O 2 |
| IUPAC Name | 2, 2-dichloroacetic acid |
| Molar Mass | 128.94 g/mol |
| Density | 1.563 g/cm3 |
| Appearance | Colourless liquid |
| Odor | Pungent odour |
| Melting point | 9 to 11 °C |
| Boiling point | 194 °C |
| Flash point | 113 °C – closed cup |
| Solubility | Miscible in water, ethanol, and diethyl ether |
Also Check – Theoretical Yield Formula
Uses of Dichloroacetic Acid
Our use of both DCA and TCA extends beyond cosmetic treatments such as tattoo removal and chemical peels. These chemicals also serve as topical medication for chemoablation, effectively targeting growths like genital warts. However, it's important to note that while they are effective in destroying abnormal cells, they can also harm healthy ones. This was evident in a randomized experiment on children with congenital lactic acidosis, where DCA showed positive clinical outcomes without causing significant adverse effects. Yet, a separate experiment on MELAS syndrome patients had to be stopped due to severe nerve toxicity experienced by all 15 participants treated with DCA. In other applications, we utilize DCA in the production of other chemicals as well as using it as a disinfectant byproduct in water purification. Medical professionals have also utilized DCA in chemotherapy for skin cancer and researchers are exploring its potential benefits in treating diabetes.
Also Check – Tyndall Effect Formula
Hazards
- Inhaling DCA can cause wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest.
- Skin burns and eye damage may result from contact with its molten form.
- The corrosive chemicals in DCA will destroy the membranes of the mouth, throat, and oesophagus if consumed.
- Additionally, it can damage the lungs, liver, pancreas, kidneys, and brain.
- When heated, it emits toxic fumes of chlorides. It is very toxic to aquatic life.
Dichloroacetic Acid Formula FAQs
Q1. What is the chemical formula of Dichloroacetic Acid (DCA)?
Q2. What are the common uses of Dichloroacetic Acid?
Q3. Is Dichloroacetic Acid safe for human consumption?
Q4. How is Dichloroacetic Acid different from Trichloroacetic Acid (TCA)?
Q5. What precautions should be taken when handling Dichloroacetic Acid?










