
Important Questions for Class 11 Physics Chapter 11: Chapter 11 of Class 11 Physics, Thermodynamics, focuses on the principles governing heat, work, and energy in physical systems. Key concepts include the laws of thermodynamics, the first law (conservation of energy), and the second law (entropy).
The chapter covers topics such as internal energy, work done in thermodynamic processes, heat transfer, and the efficiency of heat engines. Important derivations include the work done in isothermal and adiabatic processes and the concept of reversible and irreversible processes. Understanding these principles is essential for solving problems related to energy conservation, engines, and entropy in thermodynamic systems.Important Questions for Class 11 Physics Chapter 11 Overview
Chapter 11 of Class 11 Physics, Thermodynamics, is crucial for building a strong foundation in understanding energy, heat, and work in physical systems. Key topics include the laws of thermodynamics, work and heat in different processes (isothermal, adiabatic), and the concept of entropy. Important questions often focus on applying the first and second laws to various thermodynamic processes, understanding the derivations of work done, and calculating the efficiency of heat engines. These concepts are vital not only for Class 11 exams but also for higher studies and competitive exams like JEE, as they form the basis for energy-related applications in physics and engineering.Important Questions for Class 11 Physics Chapter 11 PDF
In this section, we have provided a PDF containing important questions from Class 11 Physics Chapter 11, Thermodynamics. These questions cover key concepts such as the laws of thermodynamics, work done in various processes, entropy, and the efficiency of heat engines. Solving these questions will help strengthen your understanding of the chapter and improve your problem-solving skills, which are essential for exams and competitive tests.Important Questions for Class 11 Physics Chapter 11 PDF
Important Questions for Class 11 Physics Chapter 11 Thermodynamics
Below is the Important Questions for Class 11 Physics Chapter 11 Thermodynamics -1. If an air is a cylinder is suddenly compressed by a piston. What happens to the pressure of air?
Ans: If the piston suddenly compresses then it causes heating and rise in temperature and if the piston is maintained at same Position, then the pressure falls as temperature decreases.
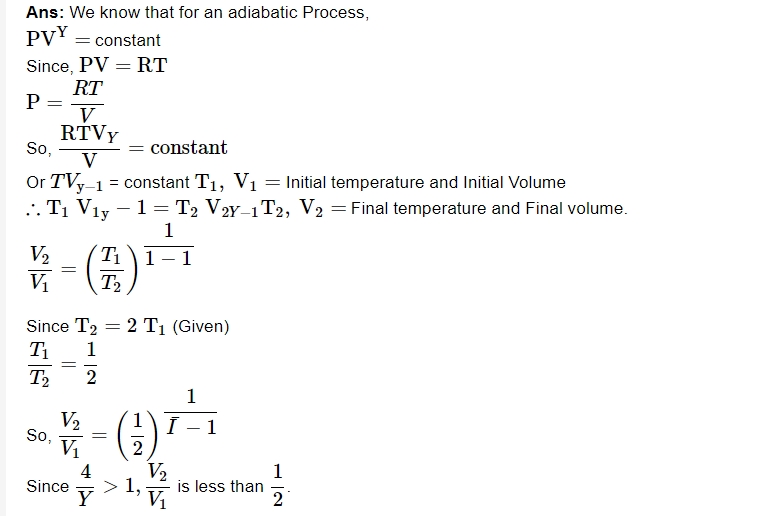
2. What is the ratio of find volume to initial volume if the gas is compressed adiabatically till its temperature is doubled?
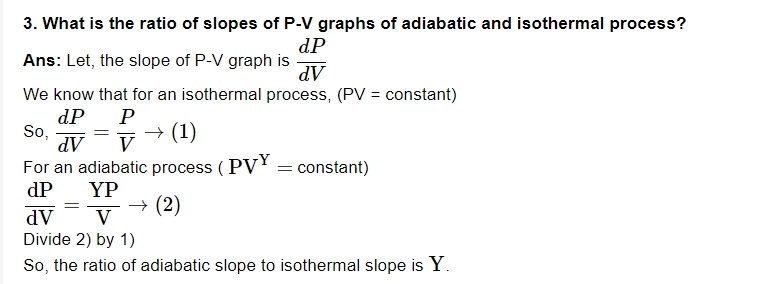
4. What is the foundation of Thermodynamics?
Ans: The law of conservation of energy and the observation that heat travels from a hot body to a cool body are the foundations of thermodynamics.
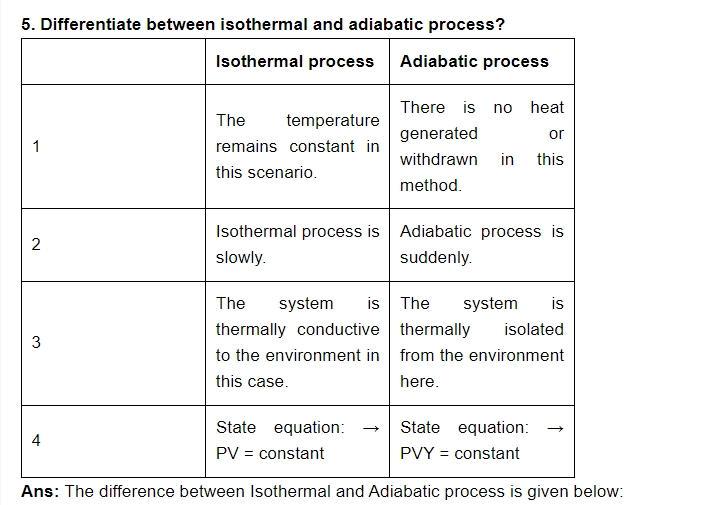
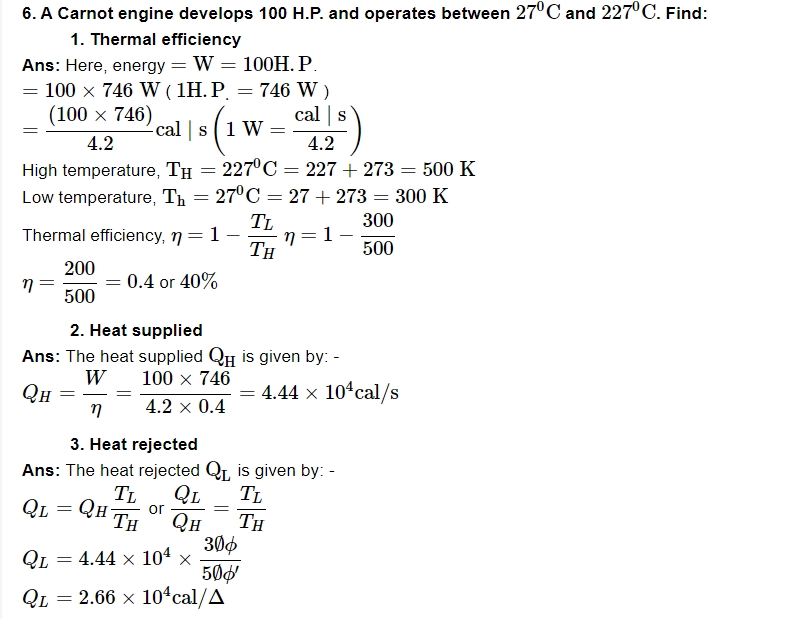
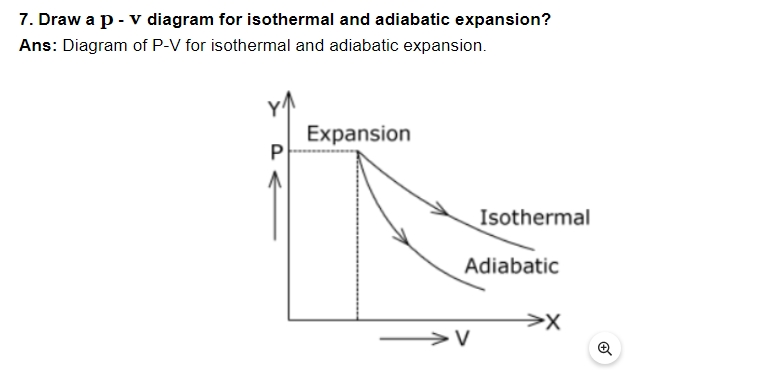
8. State zeroth law of thermodynamics?
Ans: According to Zeroth law, if the thermodynamic system and are each in thermal equilibrium with a third thermodynamic system C, then the system and are also in thermal equilibrium.
9. Can a gas be liquefied at any temperature by increase of pressure alone?
Ans: No, only when the temperature of the gas is below its critical point can it be liquefied by pressure alone.
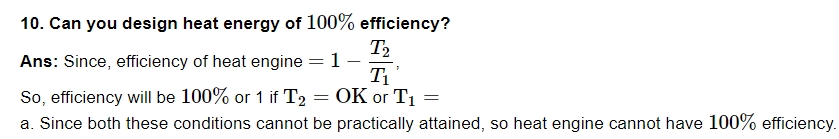
11. If air is a bad conductor of heat, why do we not feel warm without clothes?
Ans: We do not feel warm without clothes because, when we are without clothes air carries away heat from our body due to convection and we feel cold.
12. A body with large reflectivity is a poor emitter why?
Ans: This is because a body with large reflectivity is a poor absorber of heat and poor absorbers are poor emitters.
13. Animal’s curl into a ball, when they feel very cold?
Ans: When animals curl, their surface area decreases, and because energy radiated varies directly with surface area, heat loss due to radiation is reduced as well.
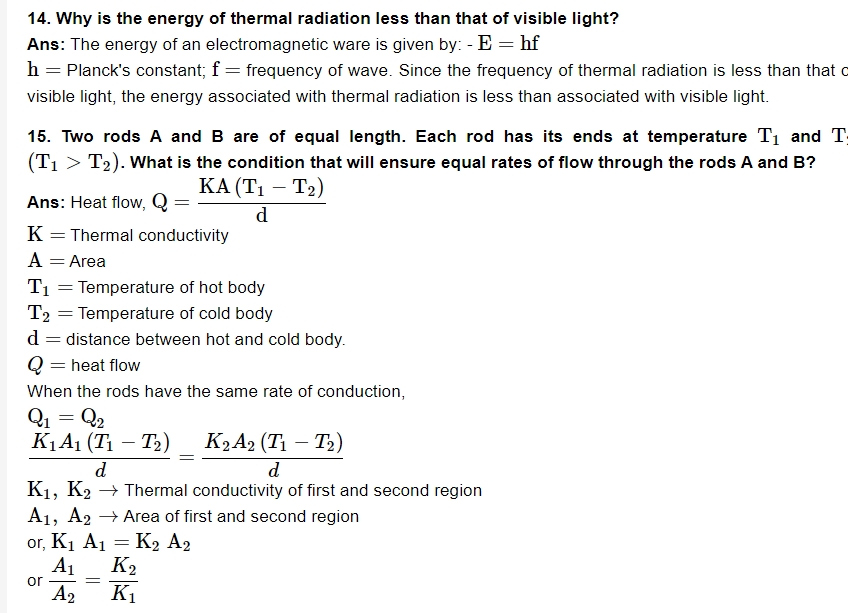
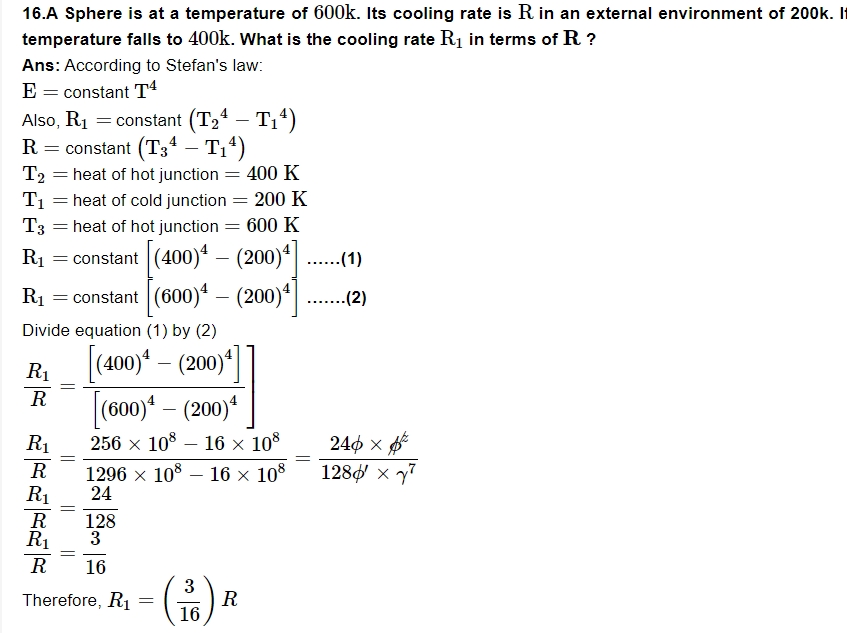
18. On a winter night, you feel warmer when clouds cover the sky than when sky is clear. Why?
Ans: We know that earth absorbs heat in day and radiates at night. When sky is covered, with clouds, the heat radiated by earth is reflected back and earth becomes warmer. But if sky is clear the heat radiated by earth escapes into space.


21. Why is latent heat of vaporization of a material greater than that of latent heat of fusion?
Ans: When a liquid turns into a gas, the volume expands dramatically, and a significant amount of work is required against the surrounding atmosphere. The heat connected with the transition from solid to gas is known as latent heat of vaporisation, and therefore the answer.
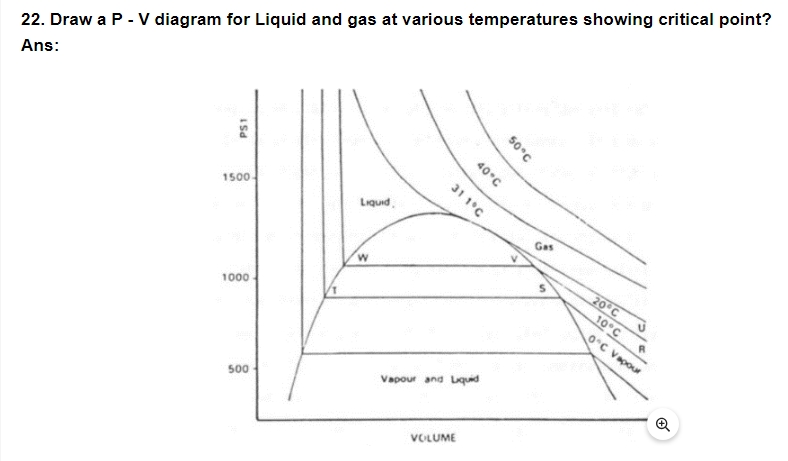
23. Why is temperature gradient required for flow of heat from one body to another?
Ans: Temperature gradient is required because, Heat flows from higher temperature to lower temperature. Therefore, temperature gradient (i.e., temperature difference) is required for the heat to flow one part of solid to another.
24. Why are Calorimeters made up of metal only?
Ans: Calorimeters are made of metal because metal is a good conductor of heat and thus allows for quick heat exchange, which is essential for calorimeter operation.
25. If a body has infinite heat capacity? What does it signify?
Ans: The term "infinite heat capacity" refers to a substance's ability to maintain its temperature regardless of how much heat it receives or loses.
26. Define triple point of water?
Ans: In all three states of matter, the triple point of water represents the pressure and temperature values at which water coexists in equilibrium.
27. State Dulong and petit law?
Ans: According to Dulong and petit law, the specific heat of all the solids is constant at room temperature and is equal to 3 R.
28. Why the clock pendulums are made of invar, a material of low value of coefficient of linear expansion?
Ans: Clock pendulums are made of invar Because, the clock pendulums are made of Inver because it has low value of a (co-efficient of linear expansion) i.e., for a small change in temperature, the length of pendulum will not change much.
Benefits of Using Important Questions for Class 11 Physics Chapter 11
Important Questions for Class 11 Physics Chapter 11 FAQs
What is the main rule of thermodynamics?
What is thermodynamics used for?
What is the basic first law of thermodynamics?
What are the objectives of thermodynamics?









