
CBSE Class 11 Chemistry Notes Chapter 12: The fundamental ideas in the field of organic chemistry are covered in this chapter. The fundamental study of organic molecules is also covered. You must have a firm grasp of these fundamental ideas before going on to more complex topics in organic chemistry. As per the CBSE exam pattern, the Class 11 Chemistry syllabus is divided into theory and practical components, with the theory paper carrying 70 marks and practicals contributing 30 marks.
Students will have a strong basis from which to develop their comprehension of the difficult organic chemistry formulas and procedures in the upcoming chapters.Our class 11 chemistry chapter 12 notes will help you understand the chapter and the ideas that are required to understand the following chapters in organic chemistry. The CBSE Class 11 Chemistry Notes Chapter 12 are very important for competitive exams like NEET and JEE.
Class 11 Chemistry Chapter 12 Organic Chemistry Some Basic Principles & Techniques
"Organic Chemistry" was first used by Berzelius in 1807 to refer to the study of substances that are obtained from natural sources.Before transforming into products, bonds are typically formed and broken in a series of discrete steps. The mechanism of the reaction is a detailed sequential description of all the steps.
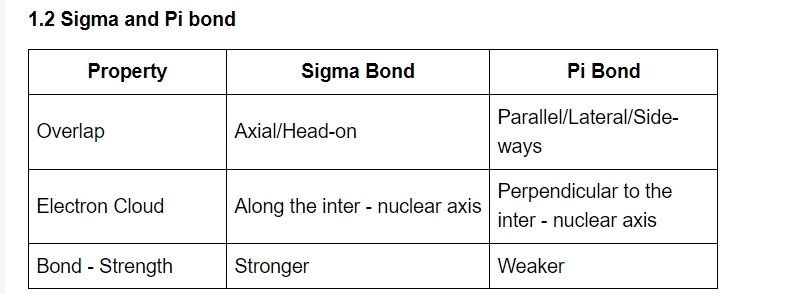
1.3 Structural Formulas
Organic chemists use a variety of formulas to represent organic compounds.
1.3.1 Complete Formulas
Lewis structures are used to represent all bond pairs of electrons as a dash (–) in complete formulas. A lone pair of electrons is represented by a pair of dots.
1.3.2 Condensed Formulas
Condensed formulas do not include all of the individual bonds. Each central atom is depicted, along with the atoms that are bonded to it.
1.3.3 Line-Angle Formulas
They are sometimes known as stick figures or skeleton formations. For cyclic compounds and, in rare cases, non-cyclic compounds, line-angle formulas are commonly utilized.
Bonds are represented by lines, and it is considered that carbon atoms are present at the intersection of two lines or the beginning or end of a line. In the majority of these illustrations, hydrogens are implied.
1.3.4 Tetrahedral Representation
This is how molecules are generally represented in three dimensions (3-D). A dashed or solid wedge indicates a bond that projects behind the plane, away from the observer, or out of the plane, towards the observer. A normal line (—) is used to depict bonds in the paper's plane.
Degrees of Carbon
It is defined as the number of carbons attached to the carbon under study.
Hybridization
The process of hybridization occurs when two or more atomic orbitals of similar energy combine in the valence shell of an atom (the center atom of a molecule or an ion) to form new, degenerate orbitals that are referred to as hybrid orbitals.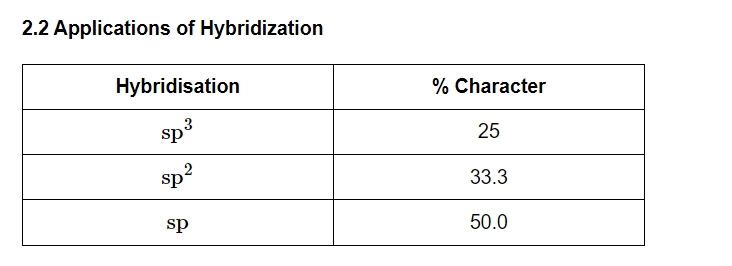
Size of Hybrid Orbitals
The size of the hybrid orbital decreases as the percentage of s-characters increases. As a result, the size of the hybrid orbital is sp3 > sp2 > sp.Electronegativity of Hybrid Orbitals
As the percentage of s-characters increases, so does the electronegativity of the hybrid orbital. As a result, the EN of the Hybrid Orbital is sp > sp2 > sp3
Dienes
Dienes are organic compounds that have two double bonds in them. Dienes are classified into three types: Isolated, Conjugated, and Cumulated.
Isolated Diene
In this case, double bonds are separated by at least one sp3 carbon.
Commonly occurring forms of carbon
Carbon's most common forms are
(a) diamond,
(b) graphite
(c) carbides.
(d) Fullerenes
(e) Charcoal
Breaking of Bonds
The bond that is important for the study of reactions in organic chemistry is the covalent bond. As a result, we investigate how a covalent bond can be broken.
(a) Homolytic Fission
(b) Fission by Heterolysis
Homolytic Fission or Homolytic Cleavage
In this kind of bond breaking, each atom splits off with one electron, creating the very reactive species known as radicals (or free radicals). Two fishhooks or half-headed arrows stand for the breaking of bonds. A half-headed arrow represents the movement of one electron. Strange neutral electron species are called radicals.Inductive Effect
The electron pair that forms the sigma bond in a covalent link between two dissimilar atoms is never shared evenly by the two atoms; instead, it is displaced slightly in the direction of the more electronegative species. Three different kinds of groups or atoms can be joined to carbon, as shown. Even though C has a greater electronegativity than H, the bond is still regarded as non-polar because of the tiny difference in electronegativity.Electron Donating and Electron Withdrawing Groups
A collection of atoms or a single atom can both produce the inductive effect. The measurement of relative inductive effects is done concerning hydrogen. Groups known as electron-donating groups (EDG) or electron-releasing groups (ERG) are thought to have a +I impact when they contribute electrons to the carbon chain. Groups known as electron-withdrawing groups (EWGs) are thought to exert the –I effect by removing electrons from the carbon chain.
Mesomeric Effect
The system's electrons carry the permanent polarisation brought about by a group conjugated with a pi bond or a set of alternative bonds, which causes a change in the unsaturated chain's electron distribution. The redistribution of electrons in unsaturated compounds conjugated with electron-releasing or electron-drawing groups (or atoms) is known as the mesomeric effect, sometimes known as the resonance effect. This impact is lasting, as indicated by the dipole moment.
Electron-Releasing Groups (+R or +M effect)
The atom connected with the conjugated system has a lone pair to donate, which is shared by all of the groups listed. As a result, a generic representation can be represented.
Electron-Withdrawing Groups (–R or –M effect)
Applications of Mesomeric Effect
Effect on Acidic Strength of Carboxylic Acids and Phenols
Compared to the charge-delocalized carboxylate ion, the charge-separated structure produced by the resonating structure of carboxylic acid is less stable. Consequently, the proton (H) that carboxylic acid needs to produce a carboxylate ion is readily lost. Similar to this, resonance in phenol leads to charge separation, which speeds up ionization and produces the phenoxide ion, which charges delocalization stabilizes.Effect on Reactivity of Carboxylic Acid Derivatives
The following graphic illustrates a typical nucleophilic reaction: Lower reactivity results from a nucleophile's difficulty breaking a bond, which increases with the strength of the link between C and Z. The carboxylic acid derivatives reactivity order concerning nucleophilic acyl substitution is: > Acid Anhydride > Ester > Amide > Acyl ChlorideClass 11 Chemistry Chapter 12 Notes PDF
Students can improve their ability to solve problems, obtain a deeper understanding of organic chemistry, and efficiently prepare for their exams by going over these notes for the Class 11 pdf. They are an invaluable resource for independent learning, editing, and test-taking.
Class 11 Chemistry Chapter 12 Notes PDF
Study without using the internet
Benefits of CBSE Class 11 Chemistry Notes Chapter 12
Simplified Content: By keeping things brief and uncomplicated, the content is delivered in a way that helps students understand difficult ideas and remember crucial information.
Practice Questions: To help students assess their comprehension and reinforce what they've learned, the notes frequently include practice questions and examples.
Accessible Resource: These notes are a handy tool for independent study and reference because students may access them from anywhere at any time.
CBSE Class 11 Chemistry Notes Chapter 12 FAQs
Which is the hardest chapter in chemistry class 11?
Which chapter is hard in chemistry?
What is the basic concept of chemistry?









