
Important Questions for Class 11 Physics Chapter 12: Chapter 12 of Class 11 Physics, "Kinetic Theory," deals with the microscopic understanding of gases. It explores the behavior of gas molecules, assuming that they are in constant random motion. Key topics include the concept of pressure, temperature, and volume as related to molecular motion, and the ideal gas laws.
The chapter introduces the ideal gas equation PV=nRT and explains concepts like mean free path, root mean square velocity, and the kinetic energy of gas molecules. Important questions focus on deriving relations for pressure, temperature, and understanding the kinetic interpretation of the gas laws.Important Questions for Class 11 Physics Chapter 12 Overview
Class 11 Physics Chapter 12 on Kinetic Theory explores the behavior of gases at the molecular level, providing essential insights into concepts like pressure, temperature, volume, and the ideal gas law. Important questions in this chapter help students grasp key principles such as the relationship between temperature and molecular motion, the kinetic theory assumptions, and the derivation of gas laws. Mastery of these questions is crucial for building a solid foundation in thermodynamics and fluid mechanics. Understanding Kinetic Theory also aids in tackling real-world applications, such as engine efficiency and weather patterns, making it a vital topic for future physics studies.Important Questions for Class 11 Physics Chapter 12 PDF
Important questions for Class 11 Physics Chapter 12 on Kinetic Theory help in understanding the behavior of gases, molecular motion, and key concepts like the ideal gas law, pressure, and temperature. These questions are essential for mastering thermodynamics and fluid mechanics, forming a strong foundation for future studies in physics. Below, we have provided a PDF with essential questions for thorough practice and deeper understanding of the chapter.Important Questions for Class 11 Physics Chapter 12 PDF
Important Questions for Class 11 Physics Chapter 12 Kinetic Theory
Below is the Important Questions for Class 11 Physics Chapter 12 Kinetic Theory -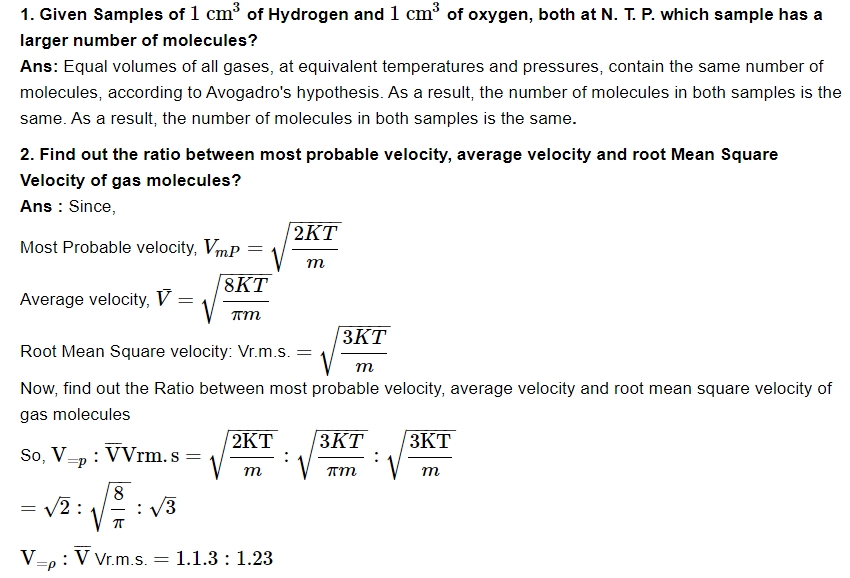
3. What is Mean free path?
Ans: The average distance a molecule travels between collisions is known as the mean free path. It is symbolised by (lambda). Meters are the units of measurement (m).
4. What happens when an electric fan is switched on in a closed room?
Ans: When an electric fan is turned on, electrical energy is first transferred into mechanical energy, which is subsequently converted into heat. Heat energy increases the kinetic energy of air molecules, raising the temperature of the environment.
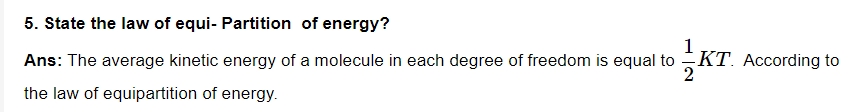
6. On what factors, does the average kinetic energy of gas molecules depend?
Ans: The absolute temperature is the only variable that affects average kinetic energy, and it is directly proportional to it.
7. Why the temperature less than absolute zero is not possible?
Ans: Since mean square velocity is proportional to temperature, it's a no-brainer. If the temperature is 0, the mean square velocity is also zero, and since molecules cannot be negative, temperatures lower than the absolute zone are not attainable.
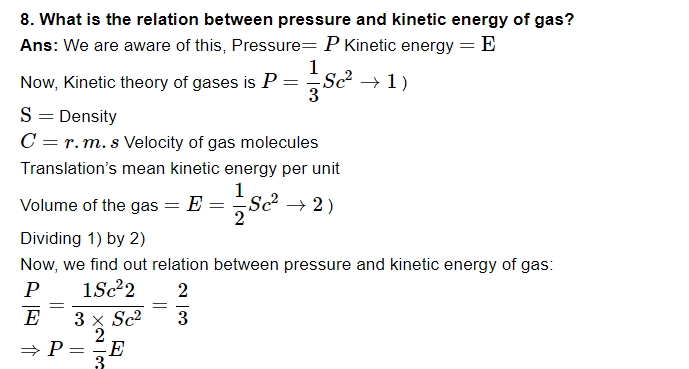
9. What is an ideal perfect gas?
Ans: Ideal gas is defined as a gas that obeys the following laws or qualities.
1) The size of a gas molecule is zero.
2) There is no attraction or repellent force between gas molecules.
2 Marks Questions
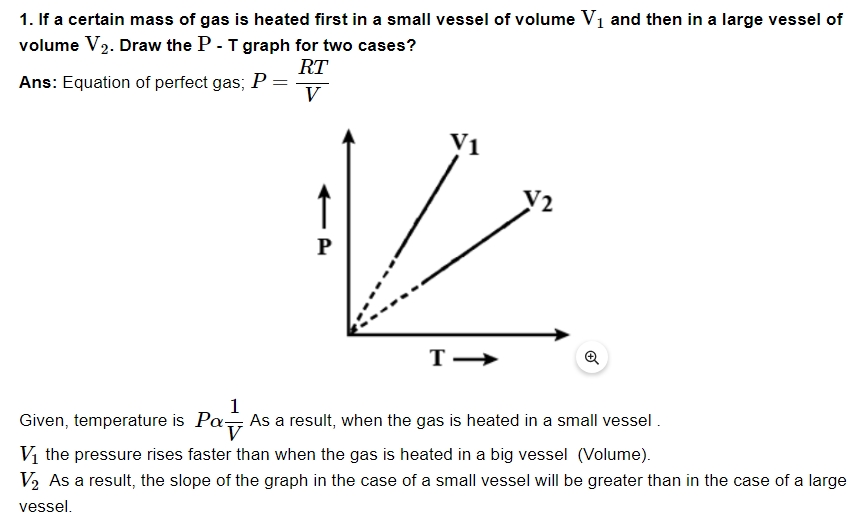


5. The earth without its atmosphere would be inhospitably cold. Explain Why?
Ans: Infrared radiation from the earth's surface is reflected back to the lower layers of the atmosphere. As a result, the earth's heat radiation from the sun is trapped by the atmosphere during the day. The earth's surface would become too cold to live if it didn't have an atmosphere.
7. At very low pressure and high temperature, the real gas behaves like ideal gas. Why?
Ans: An ideal gas has a molecule volume of zero and no intermolecular forces.
1) At extremely low pressures, the amount of gas is so huge that the volume of a molecule is insignificant in comparison to the volume of gas.
2) Because the kinetic energy of molecules is very high at very high temperatures, the effect of intermolecular forces can be ignored.
As a result, at low pressure, real gases behave like an ideal gas.

11. Air pressure in a car tyre increases during driving? Why?
Ans: Because of the action, the temperature of the air inside the Tyre rises during driving. According to Charles's law, as the temperature rises, the pressure inside the tyres rises as well.
12. What are the assumptions of kinetic theory of gas?
Ans: The following are the assumptions of the kinetic theory of gases:
1) A gas is made up of a vast number of molecules that should all be elastic spheres and identical.
2) A gas's molecules are in a constant state of rapid and unpredictable mobility.
3) Gas molecules are extremely small in comparison to the distance between them.
4) There is no attraction or repulsio between the molecules.
5) Molecule collisions with one another and with the vessel's walls are perfectly elastic.
Benefits of Using Important Questions for Class 11 Physics Chapter 12
Concept Reinforcement: Helps reinforce key concepts like molecular motion, pressure, temperature, and gas laws.
Improved Understanding: Deepens understanding of the kinetic theory and its real-world applications.
Exam Preparation: Provides targeted practice for exam preparation, ensuring familiarity with potential exam questions.
Critical Thinking: Encourages critical thinking and problem-solving skills in relation to gas behavior and thermodynamics.
Time Management: Aids in time management by allowing focused revision on the most important topics.
Foundation for Advanced Topics: Builds a solid foundation for higher-level physics topics like thermodynamics and fluid dynamics.
Important Questions for Class 11 Physics Chapter 12 FAQs
What is limitation of kinetic theory?
What are the conditions of kinetic theory?
What are the factors affecting kinetic theory?
What is the weakness of kinetic theory?









