
When acetic acid reacts with silver carbonate, Silver Acetate is formed CH 3 CO 2 Ag or AgC 2 H 3 O 2 . Silver Acetate can also be prepared by adding an acetate ion to aqueous silver nitrate. Physically, it appears as a white or greyish solid with a slightly acidic smell. Silver oxide is formed on heating, resulting in highly reflective, highly conductive silvered polymer films. Silver Acetate is a moderately water-soluble crystalline silver source that decomposes into silver oxide.
Properties Of Silver Acetate
| Chemical formula | C 2 H 3 AgO 2 |
| Molecular weight | 166.912 g/mol |
| Density | 3.26 g/cm3, solid |
| Chemical names | Silver(I) acetate, Silver monoacetate, silver (1+) salt, Silver ethanoate |
| Boiling point | Decomposes at 220 °C |
Chemical Properties of Silver Acetate
In the presence of catalysts such as nickel, palladium, or platinum, silver acetate reacts with molecular hydrogen (H2).
2CH 3 CO 2 Ag + H 2 ⇢ 2Ag + 2CH 3 CO 2 H
Silver acetate decomposes on heating to give acetone, carbon dioxide, oxygen, and silver metal.
2CH 3 CO 2 Ag ⇢ 2Ag + (CH 3 ) 2 CO + CO 2 + (1/2)O 2
Methyl acetate is formed when Silver acetate reacts with methyl bromide. The chemical reaction is shown below.
CH 3 COOAg + CH 3 Br ⇢ CH 3 COOCH 3 + AgBr
Also Check – Discovery of Proton Formula
Silver Acetate Structural Formula
When acetic acid reacts with silver carbonate at 45–60 °C, silver acetate precipitates when cooled to room temperature.
2CH 3 CO 2 H + 2Ag 2 CO 3 + 2AgO 2 CCH 3 + H 2 O + CO 2
Silver Acetate has the following structural formula.
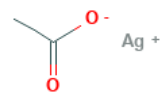
Also Check – Electrophiles Formula
Silver Acetate Preparation
Water and carbon dioxide are also produced during the reaction between Acetic acid and Silver carbonate at 45-60°C.
2CH 3 CO 2 H + 2Ag 2 CO 3 + 2AgO 2 CCH 3 + H 2 O + CO 2
By reacting Silver Nitrate with Sodium Acetate solutions, Silver Acetate is prepared.
AgNO 3 + CH 3 COONa ⇢ CH 3 COOAg + NaNO 3
Also Read – Avogadros Law
Uses of Silver Acetate
Silver Acetate can be found in a variety of forms, including:
- Anti-smoking medicines have been manufactured with Silver Acetate in the medical field.
- Silver acetate is used to prepare reflective and conductive silver polymer films.
- Acetate of silver can also be used as a pesticide.
- Chewing gum also contains silver acetate
- As a laboratory reagent, silver acetate is used.
Silver Acetate Formula FAQs
Q1. What is the chemical formula of silver acetate?
Q2. What is the appearance of silver acetate?
Q3. What is the primary use of silver acetate?
Q4. Is silver acetate toxic?
Q5. Can silver acetate be used in photography like silver nitrate?










