
Discovery of Proton was an important landmark in the history of chemistry. Protons, neutrons, and electrons are the three subatomic particles that make up an atom, the smallest unit of matter. Protons and neutrons occupy the core nucleus, and electrons circle it in predetermined orbits. These three subatomic particles were discovered in the nineteenth and twentieth centuries. Atoms combine to form molecules, and when molecules interact to produce matter (solid, liquid, or gas), molecules are the result. We shall talk about the discovery of protons and neutrons in this article, including how they were found and their characteristics.
Introduction to Proton
The subatomic particle known as a proton has a definite mass of one and a charge of one (positive charge). Either p or p+ is used as the proton's symbol. Protons are found in the nucleus of every atom. An element's atomic number is determined by how many protons it has.Also Check - Malic Acid formula
The atomic nucleus contains protons and neutrons, which are collectively called nucleons. Protons resist one another due to their positive electrical charge, but when protons and neutrons approach near enough to one another, the strong nuclear force overrides electrostatic attraction. They can join together because of this. Hadrons include both neutrons and protons. The even smaller subatomic particles known as quarks make up a proton.Discovery of Proton
Goldstein first observed positively charged particles inside an atom in 1886 using the theory that atoms are electrically neutral, meaning they have an equal quantity of positive and negative charges. A new sort of ray was created from the positive electrode (anode), which travels towards the cathode, when high voltage electricity went through a cathode tube fitted with a perforated cathode (pierced disc) carrying gas at low pressure, according to a series of tests he conducted. He called these new rays "canals," "positive," or "anode" rays.Also Checfk - Molar Volume formula
In his well-known gold foil experiment from 1909, Rutherford discovered protons. He blasted a fragile gold foil with alpha particles. Since nothing was known about the lighter nucleus, Rutherford reasoned that a hydrogen nucleus must be the fundamental component of all nuclei and maybe a brand-new fundamental particle. Rutherford suggested the hydrogen nucleus as a new particle in 1920, which he termed the proton, based on Wilhelm Wien's idea, which detected the proton in streams of ionized gas in 1898. Rutherford called it proton, derived from the Greek word "protos," which means "first."Properties Of Proton
- Protons and electrons are drawn to one another because they have opposing electric charges. Two protons repel one another because, like charges repel one another.
- The stable particle known as a proton does not disintegrate into other particles. Free protons are frequently produced when there is enough energy to detach protons from electrons.
- Plasma contains unbound protons. Protons make up around 90% of cosmic rays.
- Free neutron radioactive decay can result in the production of protons, electrons, and antineutrinos.
Also Check - Bond Order Formula
Experiment For Discovery Of Proton
Rutherford shot alpha particles onto a fragile gold foil in an experiment for the discovery of proton. Then, he detected the dispersed alpha particles on a zinc sulfide (ZnS) screen. Rutherford remarks that m ost alpha particles flowed through the foil without being deflected. A limited amount of alpha particles are deflected. One in 20,000 particles had a bounce. Rutherford made the following suggestions in light of these findings: The majority of an atom's mass and all of its positive charge are contained within a tiny core known as the nucleus. A proton is the name given to the positively charged particle. An atom's volume is primarily made up of empty space. The number of positively charged and negatively charged electrons scattered outside the nucleus equals. [caption id="attachment_16131" align="alignnone" width="300"]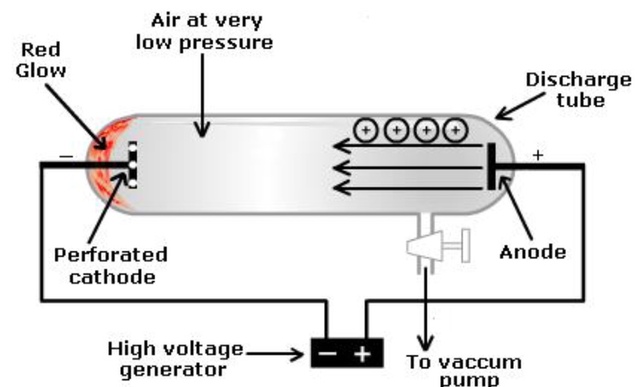 Discovery of Proton[/caption]
Discovery of Proton[/caption]
Important Facts About Discovery Of Proton
The Greek word "proton" refers to "first." The phrase was originally used in 1920 by Ernest Rutherford to refer to the hydrogen atom's nucleus. Despite not having a name until the 20th century, William Prout proposed the proton's existence in 1815. Various proton examples: There are free protons. A proton is, for instance, the nucleus of a hydrogen atom or the H+ ion. No matter the isotope, every hydrogen atom has one proton, every helium atom has two, every lithium atom has three, etc.Discovery Of Proton and Neutron Formula FAQs
How did Goldstein find out about the proton?
Who gave proton its original name?
Which atoms contain protons?
Explain the molar volume formula unit.
What materials make up protons?










