

Urea Formula: Urea, also known as carbamide, is a nitrogenous compound commonly produced as a waste product of protein metabolism in both animals and humans. Its discovery dates back to 1727 when Dutch chemist Herman Boerhaave identified it. In 1828, German chemist Friedrich Wohler achieved a significant milestone by synthesizing urea for the first time. He accomplished this by converting ammonium cyanate, an inorganic compound, into an organic compound sourced from animal materials. Urea earned its alternative name, carbamide, due to the presence of specific groups—a carbonyl group attached to two amide groups. This carbonyl group involves a carbon atom double-bonded to an oxygen atom.
Urea plays a vital role as the primary component of human urine. The liver transforms ammonia into urea, which subsequently enters the bloodstream. The kidneys then filter urea and other waste compounds from the blood, and these waste products, along with urea, are eventually excreted as urine through the urinary tract.
Physically, urea presents as a colorless to white crystalline solid and finds extensive use as a fertilizer. Its density is 1.32 g/cm³. Urea is a nitrogen-containing compound naturally present in urine and is synthesized through the urea cycle. Beyond its role in agriculture, urea serves as a raw material for animal feed. It boasts a melting point of 134°C and exhibits the ability to absorb moisture from the surrounding environment. Moreover, it is highly soluble in water and can be represented by the chemical formula CO(NH₂)₂. However, it's worth noting that long-term exposure to urea can be detrimental to the skin.
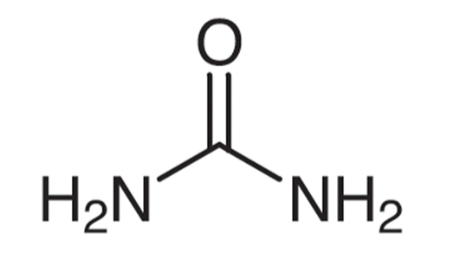
The chemical formula for urea is CO(NH₂)₂, with a molar mass of 60.06 g/mol. This compound consists of carbon, nitrogen, oxygen, and hydrogen, featuring two amide groups (-NH₂) bonded to a carbonyl group, where a carbon atom is double-bonded to an oxygen atom (-C=O).
Structure of Urea
Urea maintains a planar geometry, meaning all of its atoms lie in the same plane. It is characterized by covalent bonds, where electrons are shared between atoms, resulting in nonpolar bonds between carbon and oxygen, as well as carbon and nitrogen.
Physical Properties of Urea
- Molar mass: 60.06 g/mol.
- Appearance: Colorless to white crystalline solid.
- Density: 1.32 g/cm³.
- Melting point: 134°C.
- Solubility: Easily soluble in water and alcohol.
- Odor: Odorless.
- Insoluble in ethane.
- Weak base.
Chemical Properties of Urea
Reaction with alkaline sodium hypochlorite produces hydrazine:
CO(NH₂)₂ + NaOCl + 2NaOH → N₂H₄ + NaCl + Na₂CO₃ + H₂O
Hydrolysis with water generates isocyanate and ammonia:
CO(NH₂)₂ + H₂O → HNCO + NH₃
HNCO further hydrolyzes to produce ammonia and carbonic acid:
HNCO + NH₃ → H₂CO₃ + 2NH₃
Urease-urea reaction leads to hydrolysis into ammonia and ammonium carbamate, which then further hydrolyzes to produce another ammonia molecule and carbonic acid.
Reaction with sodium hydroxide releases ammonia and sodium carbonate:
NH₂CONH₂ + 2NaOH → 2NH₃ + Na₂CO₃
Reacting with nitric acid results in the production of urea nitrate, a high-explosive compound:
HNO₃ + (NH₂)₂CO → (NH₂)₂COHNO₃
Methods of Preparing Urea
Ammonia and Carbonyl Chloride Reaction: Urea can be synthesized in the laboratory by reacting ammonia with carbonyl chloride:
COCl₂ + 2NH₃ → CO(NH₂)₂ + 2HCl
Ammonia and Carbon Dioxide Reaction: Urea can also be prepared by mixing liquid ammonia with liquid carbon dioxide to form ammonium carbamate. Heating ammonium carbamate at high pressure and temperature yields urea:
2NH₃ + CO₂ → NH₂COONH₄ → CO(NH₂)₂ + H₂O
Ammonia and Alkyl Carbonates or Diethyl Carbonate: Another method involves the reaction of ammonia with alkyl carbonates or diethyl carbonate:
(C₂H₅O)₂CO + 2NH₃ → CO(NH₂)₂ + 2C₂H₅OH
Dehydration of Carbamate: Urea can be obtained through the dehydration of carbamate:
NH₂CO₂NH₄ ⇌ (NH₂)₂CO + H₂O
Harmful Effects and Safety Measures
Urea can decompose at high temperatures, emitting toxic fumes and causing eye and respiratory irritation.
It reacts vigorously with compounds like nitrates and perchlorates.
Prolonged contact with the skin can be harmful, so avoid contact with eyes, skin, and clothing.
Empty urea containers may still contain hazardous residues, so handle them with care and keep them away from incompatible and combustible materials.
Uses of Urea
Urea is used in explosives, skincare products for moisturizing, and as a fertilizer in agriculture. It's also a key ingredient in animal feed, the production of hydrazine, and various chemicals for pest control. In laboratories, urea helps study proteins, and it's a building block for making adhesives and plastics like urea-formaldehyde. Its versatility makes it vital in several industries.
Urea Formula FAQs
What is urea used for?
Is urea harmful to the skin?
How is urea synthesized?
What role does urea play in agriculture?
Can urea be found in cosmetics?












