
Vinegar Formula: Many of us are likely familiar with vinegar, a commonly used household item. However, in this article, we will explore some lesser-known aspects of vinegar. Vinegar, chemically known as acetic acid or ethanoic acid (CH 3 COOH), is a solution consisting primarily of acetic acid. It is widely employed in various culinary and domestic applications.
Vinegar is produced through different methods, including traditional approaches like the Orleans Process, which utilizes oak casks, and the Generator Process, which involves surface culture. Its versatility extends beyond the kitchen, as vinegar serves as an effective cleaning agent and finds use in several medicinal applications as an antiseptic.
So, what exactly is vinegar? Vinegar is a compound with the chemical formula CH 3 COOH, also referred to as ethanoic acid. Its production involves a two-step fermentation process. Initially, yeast consumes the sugars or starches present in plant-based liquids such as fruits, potatoes, or rice, leading to the formation of alcohol. The vinegar we commonly encounter typically contains 5 to 8 percent acetic acid.
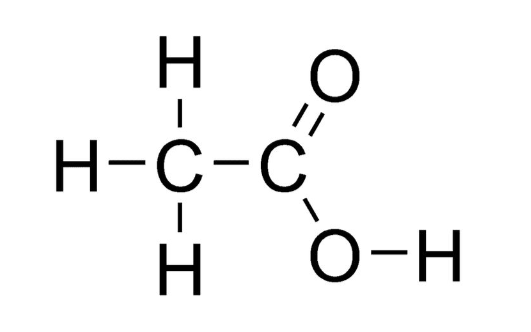
Speaking of the chemical formula of vinegar, it's important to note that vinegar is a solution comprising various compounds, rather than a single chemical entity. The key component responsible for vinegar's characteristic odor and acidity is ethanoic acid, with a chemical formula of C 2 H 4 O 2 or, in a more descriptive structural formula, CH 3 COOH.
In the structural formula, the first carbon atom is bonded to three hydrogen atoms, while the second carbon atom is linked to a hydroxyl group and an oxygen atom. This arrangement results in the presence of a functional group known as a carboxyl group (-COOH) in acetic acid's molecular structure.
Vinegar Preparation Methods
Orleans Process: This method is characterized by its slow pace. It involves filling large barrels with a mixture of wine and vinegar, allowing Acetic Acid Bacteria to ferment it gradually. After one to three months, the matured vinegar is extracted, and fresh wine is added. To ensure sufficient air for the bacteria, the barrel is only filled halfway. Over time, the bacteria acidify the mixture, leading to the formation of vinegar. This process demands patience due to its time-consuming nature.
Quick Process : In contrast to the time-consuming Orleans process, this method offers a quicker alternative. It employs a fermenting chamber containing the necessary bacteria and other compounds. Alcoholic substrate is sprayed into this chamber, initiating the reaction that results in vinegar formation. Although heat is generated during this process, air is introduced into the chamber to maintain a cool environment. These steps are repeated multiple times to achieve the desired quantity of vinegar.
Biological Production Of Vinegar
Vinegar is typically produced through the fermentation of ethyl alcohol or sugars into acetic acid by a type of bacteria known as acetic acid bacteria. This method involves two steps: first, fermentable sugars are transformed into ethanol through the action of yeast, and in the second step, acetic acid bacteria oxidize the ethanol into acetic acid in an anaerobic process. Vinegar typically contains four to six percent acetic acid and 94 to 97 percent water.
Properties of Vinegar
- Molar mass: 60.052 g/mol
- Density: 1.05 g/ml
- Melting point: 16°C
- Boiling point: 118°C
- Chemical formula: CH3COOH
- Appearance: Colorless liquid with a pungent vinegar-like odor and a sour taste
Reaction with Phosphorous Pentachloride (PCl 5 ):
CH 3 COOH + PCl 5 → CH 3 COCl + POCl 3 + HCl
Reaction with Phosphorous Tetrachloride (PCl 3 ):
3 CH 3 COOH + PCl 3 → 3 CH 3 COCl + H 3 PO 3
Harmful Effects of Vinegar
Concentrated vinegar can cause pain, irritation, and burns in the mouth, throat, and stomach.
Vinegar contains tyramine, which can contribute to health issues like high blood pressure, joint pain, irritable bowel syndrome, urticaria, and headaches.
Mixing vinegar with bleach produces chlorine gas, which is extremely dangerous and can cause skin burns, breathing difficulties, and more.
Safety Measures for Vinegar
- Avoid contact with eyes and skin.
- Prevent inhalation of vapors.
- Use protective clothing during handling.
- Thoroughly wash hands after handling.
- Ensure adequate ventilation.
- Wash contaminated clothing before reuse.
- Keep vinegar out of reach of children and pets.
Uses of Vinegar
Vinegar serves as a versatile household aid, finding its application in various cleaning and culinary tasks. Its acidic properties make it effective in removing stubborn grease, unsightly mold, and pesky mineral deposits. When it comes to kitchen maintenance, vinegar comes to the rescue by effortlessly cleaning microwaves and erasing crayon marks from surfaces. It's also the go-to choice for restoring the gleam of stainless steel appliances and eliminating tarnish from copper and brass items. In the realm of cleaning, vinegar acts as a trusty soap scum remover and a candle wax dissolver. Beyond its cleaning prowess, vinegar plays a prominent role in the culinary world, enhancing flavors as a key ingredient in salad dressings and cooking.
Vinegar Formula FAQs
What is vinegar made of?
How is vinegar produced?
What are some properties of vinegar?
Are there any harmful effects of vinegar?
Can vinegar react with other chemicals?










