
SSC Worksheet for chapter-2 Periodic Classification of Elements class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of SSC Board Worksheet for Class 10 Chemistry . Students of Class 10 Chemistry can get a free Worksheet for Class 10 Chemistry in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Before solving the Worksheet for class 10 one must read the textbook of your school board exam and try to read the notes given in Physics Wallah class 10 notes sections. Students can also download free pdf of Class 10 Chemistry Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
Physics Wallah worksheets for class 10 SSC can help students practice with tests and quickly revise lessons learned in the classroom with essential resources such as video lessons, short notes, and sample papers.
Class 10 Board is one of the most important year for every student and need a good planning and right study material to score good marks in your final exam. If any students need to take the online test to check their concepts or undertstanding then they can visit Chemistry Quiz for Class 10 .
Objective Type Questions
Q1. Which of the following statements is not a correct statement about the trends when going from left to right across the periodic table ?
- The elements becomes less metallic in nature
- The number of valence electron increases
- The atoms lose their electron more easily
- The oxide become more acidic
Q2. Arrange in increasing order of metallic character
- Ga > Ge > As > Se > Br
- Br > Se > As > Ge > Ga
- Ge > As > Ga > Se > Br
- Ga > As > Ge > Br > Se
Q3. Up to which element law of octaves was found to be applicable
- Oxygen
- Calcium
- Cobalt
- Potassium
Q4. Which of the following statements about the modern periodic table is correct?
- It has 18 horizontal rows known as periods
- It has 7 vertical columns known as periods
- It has 18 vertical columns known as groups
- It has 7 horizontal rows known as groups
Q5. Arrange the following elements in the order of their increasing non-metallic characters? Li, O, C, Be, F
- F < O < C < Be < Li
- F < O < Be < C < Li
- Li < Be < C < O < F
- F < O < C < Li < Be
Q6. Which of the following set of elements is written in order of their increasing metallic character
- Be, Mg, Ca
- Mg, Al, Si
- C, O, N
- Na, Li, K
Q7. On moving from left to right in a period in the periodic table, the size of the atom
- Increases
- Decreases
- Does not changes appreciably
- First decreases than increases
Q8. Which one of the following does not increase while moving down the group of the periodic table?
- Atomic radius
- Metallic character
- Valence
- No of shells is an element
Q9. Which one of the following is not triad?
- Cl, Br, I
- S, Se, Te
- C, Na, K
- Ca, Sr, Ba
Q10. Which of the following element will form an acidic oxide.
- An element with atomic number 7
- An element with atomic number 3
- An element with atomic number 12
- An element with atomic number 19
Subjective Type Questions
Q11. “Hydrogen occupies a unique position in modern periodic table” justify the statement.
Q12. Give an account of process adopted by mendeleef for the classification of electron how did he arrive at ‘Periodic Law’?
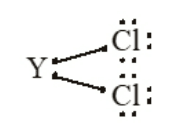
Q13. An element X with (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
- Where in the periodic table are elements X and Y placed
- Classify X and Y as metal or non-metal
- What will be nature of oxide of element Y, identify nature of bonding in compound formed.
- Draw electron dot structure of compound formed.
Q14. How could modern periodic table remove various anomalies of mendeleef’s periodic table?
Q15. What were the limitations of New land’s law of octaves?
Answer Key : Periodic Classification of Elements
|
1. (c) |
2. (a) |
3. (b) |
4. (c) |
5. (c) |
6. (a) |
7. (d) |
8. (c) |
9. (c) |
10. (a) |
Solutions
Ans 11. Hydrogen is a unique element and resembles with the elements of I A group (alkali metals) as well as those of VII A group (halogens) in its properties. It should have been placed in both I A and VII A groups but it has actually been place in I A group of periodic table.
Ans 12. Mendeleef examined the relationship between the atomic masses of the elements and their physical and chemical properties among chemical properties he concentrated on the compounds formed by elements with hydrogen and oxygen, he selected hydrogen and oxygen as they are very reactive and formed compounds with most elements the sorted out the elements with similar properties. He observed they were arranged in order of their increasing atomic masses, it was also observed that these occurs a periodic recurrence of elements with similar physical and chemical properties. On the basis he formulated a periodic law.
Ans 13. (a) X is in group 17 period 3
Y is in group 2 period 4
(b) X is a non-metal
Y is a metal
(c) The oxide will be YO and Its nature would be basic oxide the nature of bonding would be ionic bonding in the compound YO.
(d) YCl 2
Ans 14.
- The fundamental basis for modern periodic table is atomic number and net atomic mass and hence, it is more accurate.
- A separate group for noble gases could be created when noble gases were discovered.
- Hydrogen has been given a unique position in the modern periodic table at the top left corner because of its unique properties.
Ans 15.
- The law was successful up to element calcium (Ca) after that, every light element did not possess the same properties as by the element lying above it in the same group.
- One more drawback was that newland placed two elements in the same slot in a particular group.
- Newland thought that these are only so elements that existed in nature and no more elements is likely to be discovered.








