

Zinc Carbonate Formula: Zinc nanopowder finds diverse applications across various industries, including rubber manufacturing, respiratory systems, and cosmetics. The size of the powder plays a crucial role in determining the material's performance. Furthermore, owing to the growth in the cosmetic, rubber, and agriculture sectors, the utilization of zinc carbonate appears to be on the upswing in the coming years.
Zinc Carbonate
Zinc carbonate, known by various names such as smithsonite, calamine, or zinc spar, is a white crystalline powder. It was named in honor of James Smithson, a physicist. This substance is derived from zinc ore and has the chemical formula ZnCO 3 . Zinc carbonate exists in the form of a crystalline solid, sub-micron, or nanopowder. It is white, odourless, and insoluble in water, alcohol, or acetone, with slight solubility in ammonia. It has the ability to dissolve in both alkalis and acids.
Zinc carbonate serves as a significant source of zinc due to its ease of conversion into other zinc compounds like zinc oxide. This conversion process involves heating, leading to the production of zinc oxide and carbon dioxide, which is also known as calcination. Given the rapid growth in its application areas, including cosmetics, agriculture, and rubber production, the demand for zinc carbonate is on the rise. The synthesis of zinc carbonate can be achieved through various methods tailored to specific applications, production scales, or structural requirements.
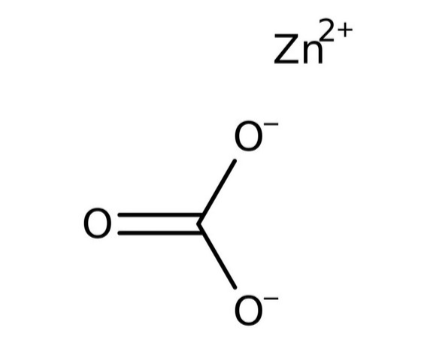
Zinc Carbonate Structure
In zinc carbonate, zinc exhibits an octahedral arrangement, with each carbonate ion forming bonds with six zinc centers, resulting in three-coordinate oxygen atoms. The chemical structure of zinc carbonate closely resembles that of calcium carbonate. The molar mass of zinc carbonate is approximately 125.4 g/mol.
Physical Properties of Zinc Carbonate
- Molecular weight: 125.38 g/mol
- Boiling point: 333.6 degrees Celsius
- Melting point: 1970 degrees Celsius
- Density: 3.5 g/cm³
- Appearance: White powder with a faint vinegar-like odor
- pH level: Greater than 10 due to its basic nature
- Solubility: Insoluble in water
Chemical Properties of Zinc Carbonate
Reaction with Acids
When zinc carbonate reacts with acids, it produces zinc chloride and releases carbon dioxide gas.
ZnCO 3 + HCl → ZnCl 2 + CO 2
ZnCO 3 + H 2 SO 4 → ZnSO 4 + CO 2 + H 2 O
Decomposition Reaction
Heating zinc carbonate results in its decomposition into zinc oxide and the release of carbon dioxide gas.
ZnCO 3 → ZnO + CO 2
Hydrothermal Synthesis
Zinc carbonate can be synthesized hydrothermally by reacting zinc chloride (ZnCl 2 ) with potassium carbonate (K 2 CO 3 ).ZnCl 2 (aq) + K 2 CO 3 (aq) → ZnCO 3 (s) + 2KCl (aq)
Preparation of Zinc Carbonate
The preparation of zinc carbonate involves the reaction of a soluble zinc salt, such as zinc sulfate, with a sodium carbonate solution. This process results in the formation of zinc carbonate (an insoluble white precipitate) and sodium sulfate (which remains in solution). The equation representing this chemical reaction is as follows:
ZnSO 4 (aq) + Na 2 CO 3 (aq) → Na 2 SO 4 (aq) + ZnCO 3 (s)
Additionally, due to zinc's amphoteric nature, its oxide can dissolve in strong alkali solutions to create zincates:
ZnO + H 2 O + 2NaOH → Na 2 Zn(OH) 4
Another method for preparing zinc carbonate involves the reaction of zincates with carbon dioxide:
Na 2 Zn(OH) 4 + 2 CO 2 → Na 2 CO 3 + ZnCO 3 + 2 H 2 O
Uses of Zinc Carbonate
Catalyst in Organic Synthesis: Zinc carbonate finds frequent application as a catalyst in diverse organic synthesis processes.
Precursor for Zinc Oxide Production : It serves as a suitable precursor for the production of zinc oxide particles.
Rubber Industry: Zinc carbonate is used in the rubber industry as a raw material to enhance the translucency or transparency of natural rubber due to its similar refractive index.
Cosmetic and Personal Care Products: Zinc carbonate is found in a wide range of products, including bath, makeup, personal hygiene, shaving, oral care, skin care, and hair care, owing to its antibacterial and antifungal properties.
Animal Feed Additives: It is used as an ingredient in animal feed additives since zinc carbonate plays a vital role in bone development, and its deficiency can hinder animal growth.
Petroleum Industry: In the petroleum industry, zinc carbonate is employed as a sulfur absorber.
Medicinal Uses: Due to its antiseptic properties, zinc carbonate is often used in medications. It also serves as an astringent and absorbent in the treatment of irritated areas.
Zinc Carbonate Formula FAQs
What is the chemical formula for Zinc Carbonate?
What elements make up Zinc Carbonate?
What is the molar mass of Zinc Carbonate?
What is the structure of Zinc Carbonate?
Is Zinc Carbonate soluble in water?












