
Zinc Hydroxide Formula: Zinc is an elemental substance denoted by the chemical symbol Zn and an atomic number of 30. At room temperature, zinc exhibits slight brittleness, and when it undergoes deoxidation, it assumes a silvery-gray hue. Positioned as the initial member in group 12 (IIB) of the periodic table, zinc holds a distinctive place.
Hydroxide, on the other hand, is a compound encompassing one or more groups, each composed of a single atom combining with both oxygen and hydrogen, serving as a negatively charged ion. The positively charged portion of this compound typically comprises an organic group (like Guanidine or tetramethylammonium), although it is generally an ion of a metal (e.g., sodium, magnesium, or aluminum).
What Exactly Is Zinc Hydroxide?
Zinc hydroxide, chemically represented as Zn(OH) 2 , is categorized as an inorganic compound. It can also be found occurring naturally in three rare minerals: wülfingite (which is orthorhombic), ashoverite, and steatite (both tetragonal).
Zinc hydroxide, along with zinc oxide, displays amphoteric properties, akin to hydroxides of other metals such as lead, aluminum, beryllium, tin, and chromium. Consequently, it easily dissolves in alkaline solutions like sodium hydroxide, as well as in dilute solutions of potent acids such as HCl.
Regarding its formula, zinc hydroxide is a scarce natural mineral on Earth, existing as an amphoteric white solid that readily dissolves in solutions of strong acids or bases. It manifests itself in the form of three infrequently found minerals like ashoverite and wulfenite, with the chemical formula Zn(OH) 2 .
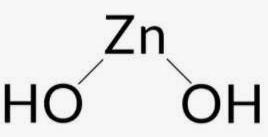
Structure of Zinc Hydroxide
Zinc hydroxide, with the chemical formula Zn(OH) 2 , exhibits a structural arrangement that can take either a tetragonal or orthorhombic form. A generalized representation of the structural configuration of zinc hydroxide is depicted below.
Preparation of Zinc Hydroxide
The synthesis of zinc hydroxide involves the creation of a sodium hydroxide solution through the addition of this alkaline compound to a solution containing any zinc salt. This process can be expressed in chemical equations as follows:
These ions are naturally encircled by water ligands, allowing zinc to dissolve in water. When an excess of sodium hydroxide is introduced, the hydroxide ions play a vital role in reducing the composite to two stages while also facilitating dissolution. The addition of ammonia to the excess creates an equilibrium that generates hydroxide ions, similar to sodium hydroxide. This process is essential for inducing the formation of the +2 charge complex with a coordination number of 4, featuring ammonia ligands.
Zn 2+ (OH 2 ) 4 (aq) + OH – (aq) → Zn 2+ (OH 2 ) 3 OH – (aq) + H 2 O (l)
Upon the addition of sodium hydroxide, the sodium hydroxide remains in solution, resulting in the formation of colorless ionized zinc water:
Formation of colorless ionized zinc water
Zn(OH) 2 + 2 OH – → Zn (OH) 4 2-
These ions are naturally encircled by water ligands, allowing zinc to dissolve in water. When an excess of sodium hydroxide is introduced, the hydroxide ions play a vital role in reducing the composite to two stages while also facilitating dissolution. The addition of ammonia to the excess creates an equilibrium that generates hydroxide ions, similar to sodium hydroxide. This process is essential for inducing the formation of the +2 charge complex with a coordination number of 4, featuring ammonia ligands.
Physical Properties of Zinc Hydroxide
- The density of zinc hydroxide is 3.05 g/cm3.
- The molar mass of zinc hydroxide is 99.424 g/mol.
- Zinc hydroxide's melting point is 125°C (257°F).
- It appears as a dull white flocculent precipitate with no discernible odor.
- Zinc in zinc hydroxide exhibits a valency of 2 and an oxidation state of +2.
- The pH of zinc hydroxide measures 8.88.
- It is slightly soluble in water and insoluble in alcohol.
Chemical Properties of Zinc Hydroxide
When aluminum reacts with a zinc hydroxide solution, it forms a white precipitate. Excess reagent leads to the formation of an Al(OH) 4 complex, indicating the presence of aluminum:
2Al 3+ (aq) + 3Zn(OH) 2 (aq) → 2Al(OH) 3 (s) + 3Zn
Zinc cations react with hydrogen sulfide in the presence of ammonia, resulting in the formation of a white precipitate of zinc sulfide. This precipitate is soluble in acids:
Zn 2+( aq) + S 2 → ZnS
Uses of Zinc Hydroxide
Zinc hydroxide finds practical applications in various fields:
- It serves as an adsorbent in medical contexts and plays a role as a fixative aid in dressing materials.
- Within the medical domain, zinc compounds are utilized in large dressings designed to absorb blood from surgical wounds.
- In industrial processes, zinc hydroxide functions as an intermediate for the production of pesticides and pigments, contributing to diverse industrial applications.
Zinc Hydroxide Formula FAQs
What is zinc hydroxide?
How is zinc hydroxide prepared?
What are the physical properties of zinc hydroxide?
What are the chemical properties of zinc hydroxide?
What are the common uses of zinc hydroxide?










