
Types Of Chemical Bonds
Chemical Bonding of Class 10
Some important types of chemical bonds are:
- Ionic or electrovalent bond (Electropositive element + Electronegative element)
- Covalent bond (Electronegative element + Electronegative element)
- Dative or Coordinate bond (Electropositive element + Electropositive element)
OCTET RULE
If two electrons are shared between two atoms, this constitutes a bond and binds the atoms together. For many light atoms, a stable arrangement is attained when the atom is surrounded by eight electrons. This octet can be made up from some electrons which are totally owned and some electrons which are ‘shared’. Thus atoms continue to form bonds until they have made up an octet of electrons. This is called the ‘octet rule’. The octet rule explains the observed valencies in a large number of cases. There are exceptions to the octet rule; for example, hydrogen is stable with only two electrons.
Ionic Bond
The bond formed between two or more atoms as a result of the transfer of one or more electron from electropositive to electronegative atom is called an ionic bond,
e.g.
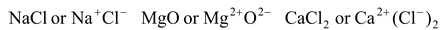 etc.
etc.
The strong electrostatic force of attraction between these oppositely charged ions is called lonic orelectrovalent bond and the number of electrons gained or lost by the atom is called its electrovalency.
Favourable conditions for forming a stable lonic bond are:
- (1) Low ionization energy of the atom forming the cation.
- (2) High electron affinity of the atom forming the anion.
- (3) High lattice energy of the crystal formed.
Characteristics of ionic compounds are :
- Each ion is surrounded by a uniformly distributed electric field i.e., each ion is non- directional. Therefore, ionic bond is also considered to be non directional. Hence ionic compounds do not show stereoisomerism.
- These are highly soluble in polar solvent, such as water and other polar solvents, but insoluble in non- polar solvents like benzene, ether, etc. (like dissolves like).
- On account of strong electrostatic attraction between the ions in the crystal of an ionic compound, these have high melting and boiling points. Order of melting and boiling points of certain compounds are examplified below.
(a) NaF > NaCl > NaBr > Nal
(b) MgO > CaO > BaO
- Ionic compounds conduct electricity in solution as well as in molten form (fused state) because ions become mobile. These free ions are able to move under the influence of an electric field and thus act as carriers of current. In solid state, they are bad conductors as the ions are tightly held.
Lattice enthalpy (or lattice energy)
The lattice enthalpy of an ionic solid is defined as energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions, i.e.,
M X (s) + lattice energy → M + (g) + X – (g)
or
M X (s) → M + (g) + X – (g), ΔH = lattice energy.
For example: The lattice enthalpy of NaCl is 788 kJ mol –1 . This means that 788 kJ of energy is required to separate to an infinite distance one mol of solid NaCl into one mol of Na + (g) and one mole of Cl – (g), i.e.,
NaCl (s) + 788 kJ →Na + (g) + Cl – (g)
or
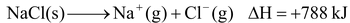 = lattice enthalpy
= lattice enthalpy
Determination of lattice Enthalpy
The direct calculation of lattice enthalpy is quite difficult because the required data is often not available. Therefore lattice enthalpy is determined indirectly by the use of the Born-Haber cycle. This cycle developed by Born and Haber, uses ionization enthalpies, electron gain enthalpies and other data for the calculation of lattice enthalpies. The procedure is based on the Hess’s law, which states that the enthalpy of a reaction is the same, whether it takes place in a single step or in more than one step. The assumption is that the formation of an ionic compound may occur either by direct combination of the elements or by an alternate process in which following steps are involved. In both the cases energy involved is the same
- The reactants are converted into gaseous state.
- The gaseous atoms are converted into ions
- The gaseous ions are combined to form ionic compound.
For example, Born-Haber cycle, for the calculation of lattice enthalpy of simple alkali metal halide ‘‘MX’’ is shown below
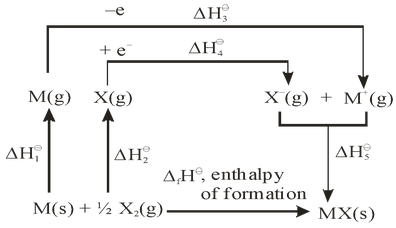
Where,
 = enthalpy of sublimation of M(s) to M(g)
= enthalpy of sublimation of M(s) to M(g)
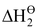 = enthalpy of dissociation of
1/2 X
2
(g) to X(g)
= enthalpy of dissociation of
1/2 X
2
(g) to X(g)
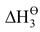 = first ionization enthalpy of M(s) to M
+
(g)
= first ionization enthalpy of M(s) to M
+
(g)
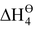 = electron gain enthalpy of X(g) to
X
-
(g)
= electron gain enthalpy of X(g) to
X
-
(g)
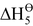 = reverse of lattice enthalpy, i.e., enthalpy of combination of M
+
(g) and X
–
(g) to produce MX(s).
= reverse of lattice enthalpy, i.e., enthalpy of combination of M
+
(g) and X
–
(g) to produce MX(s).
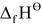 = standard enthalpy of formation of MX(s)
= standard enthalpy of formation of MX(s)
Applying Hess’s law we can write
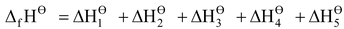 …(i)
…(i)
If we calculate
 from equation (i) and change its sign, we will get lattice enthalpy of MX(s). The following example illustrate the Born-Haber cycle.
from equation (i) and change its sign, we will get lattice enthalpy of MX(s). The following example illustrate the Born-Haber cycle.
Covalent bond
This bond is formed by mutual sharing of electrons so as to complete their octets or duplets. The number of electrons contributed by each atom for sharing is called its covalency. e.g.,
 etc.
etc.
Characteristic of covalent bond
- Under normal conditions of temperature and pressure, these exist as gases (or) liquid of low boiling points. This is due to the fact that very weak forces of attraction (Vander Waal's forces) exist between discrete molecules. Some exist as soft solids if their molecular masses are high.
- With the exception of few which have giant three dimensional structure such as diamond, carborundum (SiC), silica (SiO2), others have relatively low melting and boiling points. This is due to the presence of weak attractive forces between the molecules. On supplying heat energy, the molecules are readily pulled out from these forces and move freely having high kinetic energy.
- In general, covalent substances are bad conductors of electricity. Substances which have polar character like HCl in solution, can conduct electricity. Covalent solids having giant molecules are bad conductors since they do not contain charged particles or free electrons.
- In general, covalent substances are insoluble in polar solvents like H2O, but soluble in non polar solvents like benzene, CCl4, ether etc. This is based on the principle, like dissolves like. Some of the covalent compounds like alcohols, amines dissolve in water due to hydrogen bonding. Covalent solids having giant molecules are practically insoluble in all solvents.
- Covalent substances show molecular reactions. The reaction rates are usually low as it involves breaking and establishing of covalent bonds.
Type of covalent bond
Depending on whether the two bonded atoms differ in their electronegativites or not i.e. on bond polarity covalent bonds are of two types:
POLAR COVALENT BOND:
A covalent bond in which there is an unequal attraction for the shared electrons between the two combining atoms. Resulting in one end of the molecule becoming slightly positive and the other end becoming slightly negative is called a polar covalent bond.
A polar covalent bond is formed when two atoms having different electronegativities combine or in other words a polar covalent bond is formed between different types of atoms. likeHCl .
In HCl the electronegativity of Cl atom is more than that of H atom & thus the shared pair of electrons remains more near or attracted to Cl atom, making the H-Cl bond polar. The shifting of shared electrons from one atom to another is indicated by putting an arrow-head in the centre of the line representing the bond between the atoms. The arrow head points towards the electronegative elements (like Cl in this example).
H → Cl
Since the electrons are attracted more towards the Cl atom it acquires a slight negative charge (δδ - ) while H acquires a slight positive charge (δδ + ) as electrons move away from it. This can be represented as –
δδ + δδ -
H H ------- Cl
NON – POLAR COVALENT BOND:
A covalent bond in which there is an equal attraction for the shared pair(s) electrons between the two combining atoms is called non-polar covalent bond. A non-polar covalent bond is formed when two atoms having equal electro negativities combine or when same type of atoms combine.
e.g. Chlorine molecule
In the above example the two chlorine atoms have equal distance from both the chlorine atoms making the bond non-polar. Other examples: Fluorine molecule (F2), hydrogen molecule (H2), oxygen molecule (O2) etc.
Co-Ordinate bond
This type of bond formation occurs by one sided sharing of electrons, i.e., one atom donates a pair of electrons while the other simply shares it so as to complete its octet. The atom that donates a pair of electrons is called the donor while the other which accepts these electrons is called the acceptor. The coordinate bond is usually represented by an arrow pointing from the donor towards the accept
For example,
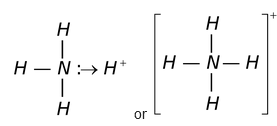
Coordinate bond is also present in SO 2 , SO 3 , O 3 , H 3 O + , NO - 3 or HNO 3 etc.
This coordinate bond has some polar character, it is also called dative or semi-polar bond.
Characteristics of coordinate compounds
1. Physical State : These exist as gases, liquids and solids under ordinary conditions.
2. Melting and boiling points : Their melting and boiling points are higher than purely covalent compounds and lower than purely ionic compounds.
3. Solubility : These are sparingly soluble in polar solvents like water but readily soluble in non-polar (organic solvents).
4. Stability : These are stable as the covalent compounds. The addition compounds are, however, not very stably. It is also a strong bond because the paired electrons cannot be separated easily.
5. Conductivity : Like covalent compounds, these are also bad conductors of electricity. The solutions or fused mass do not allow the passage of electricity.
6. Molecular reaction : These undergo molecular reaction. The reactions are slow.
7. Isomerism : The bond is rigid and directional. Thus, coordinate compounds show isomerism.
8. Dielectric Constant : The compounds containing coordinate and possess high values of dielectric constants.
Comparison of ionic, covalent and coordinate compounds.
|
Property |
Ionic |
Covalent |
Coordinate |
|
1. Binding force |
Between ions–strong (coulombic) |
Between molecules smaller (vander Waals) |
In between |
|
2. Mp/Bp |
High |
Less than ionic |
In between |
|
3. Conductor |
Conductor of electricity in fused state and in aqueous solution |
Bad conductor |
Greater than covalent |
|
4. Solubility in polar solvent (H 2 O) |
High |
Less |
In between |
|
5. Solubility in non-polar solvent (ether) |
Low |
High |
In between |
|
6. Physical state |
Generally solid |
Liquid and gaseous |
Solid, liquid and gas |










