Double Displacement Reactions
Chemical Reaction And Equation of Class 10
DOUBLE DISPLACEMENT REACTIONS
Those reactions in which two different atoms or groups of atoms are exchanged are called the double displacement reactions. These reactions generally occur between two ionic compounds in the solution. So they may be defined as :
“Those reactions in which two ionic compounds in the solution react by exchange of their ions to form new compounds are called double displacement reactions”
|
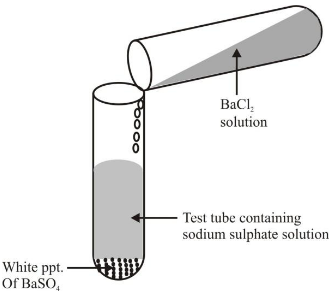
Ex 1. Reaction between Barium Chloride solution and sodium sulphate solution. Experiment: Take sodium sulphate solution in a test tube and add few drops of solution of barium chloride to it and mix the two solution. There is formation of white precipitate of Barium sulphate: The reaction is as follows : |
Formation of white ppt. of barium sulphate on adding barium chloride solution to sodium sulphate |
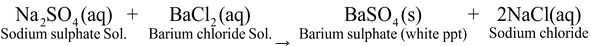
In this reaction, there is double displacement or exchange of ions, i.e. chloride ions of BaCl2 have been replaced by sulphate ions of Na2SO4 whereas sodium ions of Na2SO4 have been replaced by chloride ions of BaCl 2 . Hence it is a double displacement reaction. Above reaction is also called precipitation reaction as white precipitate of BaSO4 is formed.
|
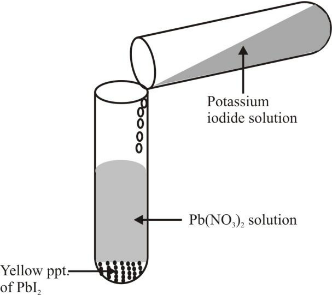
Ex 2 .Reaction between lead nitrate solution and potassium Iodide solution. Experiment: Take lead nitrate solution in a test tube and add potassium Iodide solution to it and mix the two solutions. There is formation of yellow precipitate of lead iodide. The reaction involved is :
|
Formation of yellow ppt. of lead iodide on adding potassium iodide solution to lead nitrate |

In this reaction, there is double displacement or exchange of ions, i.e. lead ions of Pb(NO3)2 have been replaced by potassium ions of KI and iodide ions of KI have been replaced by nitrate ions of Pb(NO3)2. Thus it is a double displacement reaction. Above reaction is also called precipitation reaction as yellow precipitate of PbI2 is formed.
Some more examples of double displacement reactionare :
(i)

(ii)

(iii)
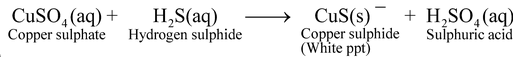
All the above reactions are double displacement reactions, as there is double exchange or displacement of ions.
Related Topics