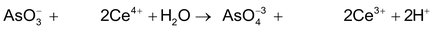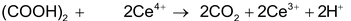Type Of Titrations
Redox Reaction of Class 11
Type Of Titrations
Acid – base or neutralization titration: The reaction in which on acid reacts with a base to give salt and water known as neutralization reaction and titration involving such a reaction known as neutralization titration.The type of indicator used to indicate end point depend on the pH of end – point.
Redox titration: Titration involving oxidation-reduction known as redox – titration. Some important example of such titration with their chemistry.
Precipitation titration: When two such solution are mixed during the course of titration a precipitate is formed and the end point is indicated by completion of precipitation.Titration involving such type of known as precipitation titration. Some common example
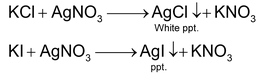
Some time titration involving silver nitrate also known as argentometric titration as all other salt of silver are insoluble in waste thus from ppt.
Complexo metric titration:
It involves the replacement of one or more of the co-ordinate covalent molecules which are co-ordinated to a central metal ion, M by some other groups. The groups getting attached to the central metal ion are known as ligand [L].

For example ethylene diamine tetra acetic acid [EDTA] is used along with eriochromic indicator.To find out the concentration or compositon of given samples titration is used in different mode named as simple titration, double titration, back titration are discussed below.
SIMPLE TITRATION
In this, we can find the concentration of a substance with the help of the conc. of another substance which can react with it.For example: Let there be a solution of a substance A of unknown concentration. We are given another substance B whose concentration is known N 1 . We take a certain known volume of A in a flask V 2 and then we add B to A slowly till all the A is consumed by B. (This can be known with the help of indicators). Let us assume that the volume of B consumed is V 1 . According to the Law of equivalents, the number of gm equivalents of A is equal to the number of gm equivalents of B.
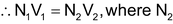 is the conc. of A.
is the conc. of A.
From this we can calculate the value of N 2 .
BACK TITRATION
Back titration is used to calculate % purity of a sample. Let us assume that we are given an impure solid substance C weighing w gms and we are asked to calculate the percentage of pure C in the sample. We will assume that the impurities are inert. We are provided with two solutions A and B, where the concentration of B is known N 1 and that of A is not known. This type of titration will work only if the following conditions are satisfied (a) A, B and C should be such compounds that A and B can react with each other, A and C can react with each other but product of A and C should not react with B.
Now we take a certain volume of A in a flask (A taken should be such that gm equivalents of A ≥ gm equivalents of C in the sample. This can be done by taking A in excess). Now we perform a simple titration using B. Let us assume that the volume of B used is V 1 . In another beaker, we again take the solution of A in the same volume as taken earlier. Now, C is added to this and after the reaction is complete, the solution is being titrated with B. Let us assume that volume of B used up is V 2 .
Gram equivalents of B used in the first titration = N
1
V
1
.
∴gm. equivalents of A initially =N
1
V
1
gm. equivalents of B used in the second titration is N
1
V
2
∴ gm. equivalents of A left in excess after reacting with C = N
1
V
2
gm. equivalents of A that reacted with C = N
1
V
1
- N
1
V
2
gm. equivalents of pure C = N
1
V
1
- N
1
V
2
If the ‘n’ factor of C is N
1
V
1
- N
1
V
2/x
∴ the weight of C = N
1
V
1
- N
1
V
2/x
× Molecular weight of C.
∴ percentage of C = N
1
V
1
- N
1
V
2/x
× Molecular weight of C./w x 100
DOUBLE TITRATION
The method involves two indicator (Indicators are substances that change their colour when a reaction is complete) phenolphthalein and methyl orange. This is a titration of specific compounds. Let us consider a solid mixture of NaOH, Na
2
CO
3
and inert impurities weighing w g. You are asked to find out the % composition of mixture. You are also given a reagent that can react with the sample, say, HCl along with its concentration (M
1
).
We first dissolve the mixture in water to make a solution and then we add two indicators in it, namely phenolphthalein and methyl orange. Now, we titrate this solution with HCl.NaOH is a strong base while Na
2
CO
3
is a weak base. So it is safe to assume that NaOH reacts with HCl first, completely and only then does Na
2
CO
3
react.

Once NaOH has reacted, it is the turn of Na
2
CO
3
to react. It reacts with HCl in two steps:
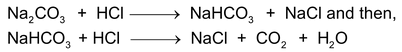
As can be seen, when we go on adding more and more of HCl, the pH of the solution keeps on falling. When Na
2
CO
3
is converted to Na
2
HCO
3
completely, the solution is weakly basic due to the presence of Na
2
CO
3
(which is a weaker base as compared to Na
2
HCO
3
. At this instant phenolphthalein changes colour since it requires this weakly basic solution to change its colour. Therefore, remember that phenolphthalein changes colour only when the weakly basic Na
2
CO
3
is present. As we keep adding HCl, the pH again falls and when all the Na
2
CO
3
reacts to form NaCl,CaO
2
and H
2
O the solution becomes weakly acidic due to the presence of the weak acid H
2
CO3(CO
2
+ H
2
O). At this instance methyl orange changes colour since it requires this weakly acidic solution to do so. Therefore, remember methyl orange changes colour only when H
2
CO
3
is present.
Now, let us assume that the volume of HCl used up for the first and the second reaction,
i.e.,
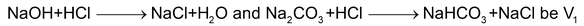
(this is the volume of HCl from the beginning of titration up to the point when phenolphthalein changes colour). Let the volume of HCl required for the last reaction,
i.e.,
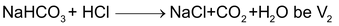
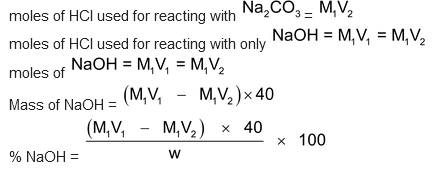
(this is the volume of HCl from the point where phenolphthalein had changed colour upto the point when methyl orange changes colour). Then, moles of HCl used for reacting with
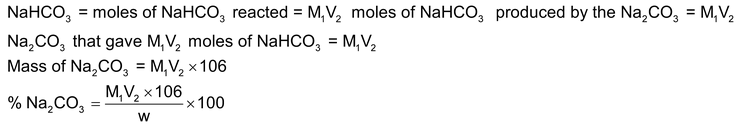
moles of HCl used for the first two reactions = M1V1
moles of Na
2
CO
3
= M1V2
Related Topics
IODOMETRIC AND IODIMETRIC TITRATION
The reduction of free iodine to iodide ions and oxidation of iodide ions to free iodine occurs in these titration
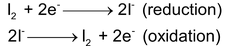
These are divided into two types
- Iodometric titration: In iodometric titrations, an oxidising agent in allowed to react in neutral medium or in acidic medium with excess of potassium iodide to librate free iodine.

Free iodine is titrated against a standard reducing agent usually with sodium thiosulphate, Halogen, dichromates, cupric ion, peroxides, etc can be estimated by this method.
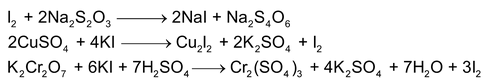
- Iodimetric titration:These are the titrations in which free iodine is used as it is difficult to prepare the solution of iodine (volatile and less soluble in water) it is dissolved in KI solution.
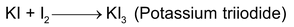
This solution is first standardised before use with the standard solution of I
2
substance such as sulphite, thiosulphate, arsenite etc. are estimated. In the iodimetric and iodometric titrations, starch solution is used as indicator. Starch solution gives blue or violet colour with free iodine. At the end point the blue or violet colour disappears when iodine is completely changed to iodide.
Potassium permanganate titration: In these titrations, reducing agents like
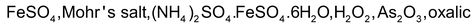 acid
acid
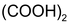 and oxalates (COONa)
2
etc. are directly titrated against KMnO
4
as oxidising agent in acidic medium.
and oxalates (COONa)
2
etc. are directly titrated against KMnO
4
as oxidising agent in acidic medium.
For Example
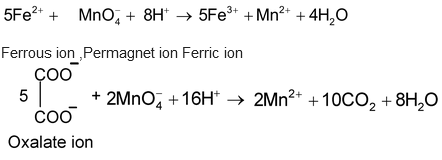
Potassium dichromate titrations
: In these titrations, the above listed reducing agents are directly titrated against K
2
Cr
2
O
7
as oxidising agent in acidic medium for example,
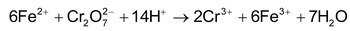
Ceric sulphate titrations
In these titrations, the reducing agents such as Fe
2+
salts,Cu
+
salts, nitrites, arsenites, oxalates etc. are directly titrated against ceric sulphate, Ce(SO
4
)
2
as the oxidising agent. For example
arsenite ion ceric ion Arsenite ion Cerous ion
Oxalic acid
Volume Strength of H
2
O
2
x volumes of H
2
O
2
means x litre of O
2
is liberated by 1 litre of H
2
O
2
on decomposition
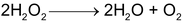
68 gm 22. 4 lit at STP
22.4 lit (at STP) of H 2 O 2 is given by 68 gm of H 2 O 2
x litre of O
2
is released from 68x/22.4 gm of H
2
O
2
= 17x/5.6
∴ x lit of O
2
is given by = 68x/22.4 = 17x/5.6 (strength)
Strength, S = 17x/5.6
Normality = S/E = 17x/5.6 x 34/2 = x/5.6 [ x = Nx5.6]
Molarity = 1/2
Normality = x/11.2
Meq. of FeSO
4
added to Mn
3
O
4
= 100 x 0.1 = 10
Meq. of FeSO
4
left after reaction with Mn
3
O
4
= Meq. of KMnO
4
used = 50 x 3/25 = 6
∴ Meq. of FeSO
4
used for Mn
3
O
4
= 10 – 6 = 4
∴ Meq. of Mn
3
O
4
= 4
∴Meq. of MnSO
4
.4H
2
O = 4
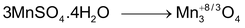
(n -factor = 8/3 – 2 = 2/3)
∴ Let weight of hydrated salt be w and molecular weight = M
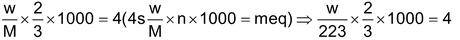
∴w = 1.338 gm