CBSE Class 8 Science Notes Chapter 4: Chapter 4 of CBSE Class 8 Science, titled "Combustion and Flames," explains the process of combustion, which is a chemical reaction involving oxygen that produces heat and light. The chapter categorizes substances as combustible and non-combustible and describes the different types of combustion, including rapid, spontaneous, and explosive combustion.
Additionally, the concept of ignition temperature is introduced, along with examples of fuels and their calorific values. The chapter emphasizes the importance of fire safety, providing measures to control fire and prevent accidents.CBSE Class 8 Science Notes Chapter 4 Overview
Chapter 4 of CBSE Class 8 Science, titled "Combustion and Flames," provides a comprehensive understanding of the process of combustion and the characteristics of flames. Combustion is a chemical reaction in which a substance (fuel) reacts with oxygen to release heat and light. Substances are classified into two categories: combustible (capable of burning) and non-combustible. The chapter explains different types of combustion, such as rapid combustion (like in gas stoves), spontaneous combustion (occurs without external heat), and explosive combustion (as seen in fireworks).CBSE Class 8 Science Notes Chapter 4 PDF Download
Here we have provided CBSE Class 8 Science Notes Chapter 4 Combustion and Flames for the ease of students. Students can access these solutions without internet by downloading them.CBSE Class 8 Science Notes Chapter 4 PDF
CBSE Class 8 Science Notes Chapter 4 Combustion and Flames
Below we have provided CBSE Class 8 Science Notes Chapter 4 Combustion and Flames -Introduction
Combustion
Combustion is the name for the chemical reaction that occurs when a material combines with oxygen to produce heat and light. One instance of combustion is the burning of wood.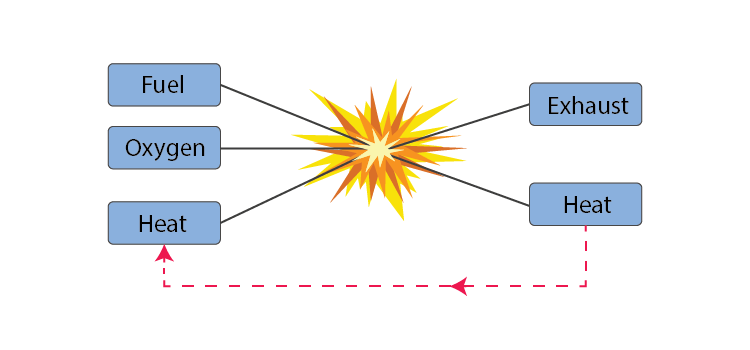
Combustible and Non-Combustible Substances
Combustible materials, like wood, coal, and paper, are substances that ignite quickly. Sand, water, and glass are examples of non-combustible materials that do not easily catch fire.History of Wood and Candle Flame
Fuel
Fuel is any substance that burns to release a usable amount of energy. For instance, nuclear energy, biogas, and fossil fuels. Fuels can exist in three different states: solid, liquid, or gas. Depending on how they occur, they might be classified as artificial or natural.Ignition Temp
The lowest temperature at which a combustible substance catches fire when heated in air is called its ignition temperature.Inflammable Substances
Inflammable compounds include acetone, fuel, and LPG. These substances have very low ignition temperatures and can readily catch fire.Fire
A chemical combustion reaction involving oxygen and a fuel source produces fire.A fire's duration is determined by the amount of fuel and oxygen that are present.Candle Flame
Fire Triangle
Three conditions must be met at the same time in order for fire to start. Some kind of fuel or flammable substance. a heat source to bring the fuel's temperature up to that needed for ignition. sufficient oxygen to keep burning. Thus, we can control the fire if we take away any one of these resources.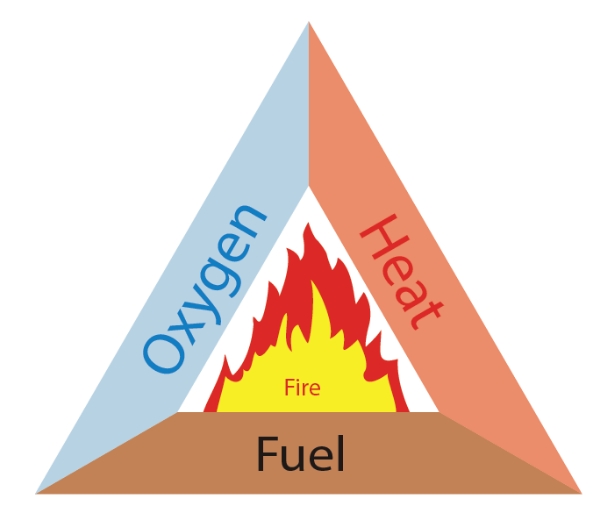
Flame
Flame is the visible and gaseous part of the fire. What we see as the flame is the light energy released due to the combustion of fuel.Zones of Candle Flame
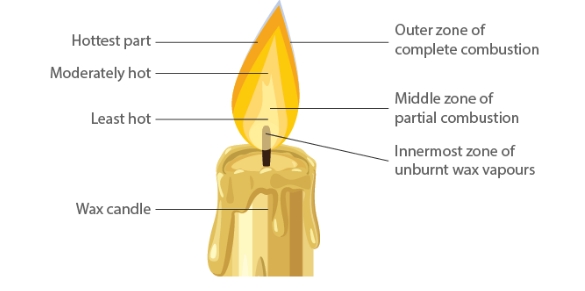
Structure of Flame
Because of total combustion, the outermost zone is the hottest of all the zones and is also blue in hue. It is the area of the flame that is not glowing. Partial fuel combustion occurs in the candle's centre zone, which is yellow in hue and somewhat hot. It is the area of the flame that is brilliant. The flame's core region is black in colour and the least heated. The reason for this is the vapours from unburned wax.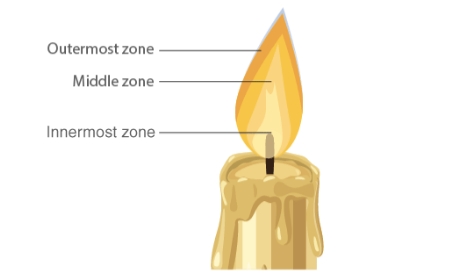
Smoke
One example of a solid (unburned particle) scattered in a gas (air) is smoke. The unburned carbon particles in the smoke are what give it its black colour.Matchstick
Types of Combustion
Rapid combustion is the kind of combustion in which heat and light are released very quickly. For instance, the burning of LPG. Spontaneous combustion is the kind of combustion that occurs when materials ignite on their own without the need for heat, as in the case of forest fires.Working of a Matchstick
Red phosphorus, which transforms into white phosphorus when heated, is the primary component of a matchstick's bulb. The matchstick begins to burn when white phosphorus spontaneously ignites, raising the wooden stem's temperature to the ignition point.Fire Extinguisher
Fire Control
Fire can be controlled by removing any or all of the factors of combustion, i.e. fuel, oxygen (air) and ignition temperature (by lowering the temperature).Fire Extinguisher
The fire brigade uses extinguishers as one of its tools for fighting fires. The purpose of fire extinguishers is to either cut off the oxygen supply or lower the fuel's temperature, or both.Calorific Value
The calorific value of a substance refers to the amount of heat energy produced when a unit mass or volume of the substance is completely burned in the presence of oxygen. It is a measure of the energy content of a fuel, determining how efficiently the fuel can release energy upon combustion. Calorific value is typically expressed in units like kilojoules per gram (kJ/g) or kilojoules per kilogram (kJ/kg) for solid and liquid fuels, and kilojoules per liter (kJ/L) or cubic meter (kJ/m³) for gaseous fuels. Higher the calorific value, the more energy the fuel can provide.Ideal Fuel
The perfect fuel is inexpensive, widely accessible, and quickly ignited. Its calorific value is high. It doesn't release any toxic gases or residues that contaminate the surroundings.Calorific Value and Efficiency of a Fuel
A fuel's calorific value is the quantity of heat energy released during the full combustion of one kilogramme of fuel. A fuel's calorific value is stated in kilojoules per kilogramme (kJ/kg). Efficiency is the percentage of energy emitted during the burning of fuel that is transformed into productive work. Its efficiency is directly correlated with its calorific value. Its efficiency will likewise be great if the value is high. Its efficiency would likewise be low if the value was low.Pollution
Pollution refers to the introduction of harmful substances or contaminants into the environment, causing adverse effects on living organisms, ecosystems, and the natural surroundings. It can occur in various forms, including air, water, soil, noise, and light pollution, each having specific sources and impacts.Harmful Products from Burning of Fuel
Respiratory issues arise from the airborne particulates of unburned carbon released during the burning of fuels such as coal, wood, and petroleum products. One of the most toxic gases produced when fuels burn partially is carbon monoxide. Carbon dioxide, which is released into the atmosphere when fuels are burned, is what causes global warming.Unburnt Carbon Particles
Wood, coal, candles, and petroleum are examples of carbon fuels that leak unburned carbon particles. These tiny particles are harmful contaminants that aggravate respiratory conditions like asthma.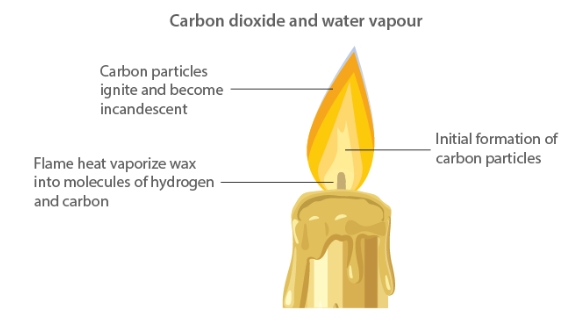
CO Emission
An incomplete burning of fuels releases the deadly chemical carbon monoxide. Burning coal in a closed space is risky because the carbon monoxide it produces can kill anyone who is sleeping there.Global Warming
Global warming is the term used to describe the increase in the average temperature of the earth's atmosphere caused by the release of carbon dioxide during the combustion of fuels. The effects of global warming include changes in rainfall patterns and the melting of polar ice caps.Acid Rain
Emissions of nitrogen oxide and sulphur dioxide combine with atmospheric water molecules to form acid, which is what causes acid showers. It seriously damages infrastructure, aquatic and terrestrial animals, and vegetation.CNG – The Clean Fuel
Because compressed natural gas (CNG) is a cleaner and less polluting fuel than diesel and petrol, it is gradually replacing these fuels in cars.Benefits of CBSE Class 8 Science Notes Chapter 4
CBSE Class 8 Science Notes Chapter 4 FAQs
What are the important points in the combustion and flame?
Heating the combustible substance to its ignition temperature. The presence of fuel plays an important role. The presence of air or oxygen. Ignition temperature is maintained (it is defined as the substance that catches fire at its lowest temperature).
What are the conditions for combustion?
Three essential conditions for the combustion of fuels are: There must be Fuel to burn. There must be Air to supply oxygen. There must be Heat (ignition temperature) to start and continue the combustion process
What is the main purpose of the combustion?
Combustion, also known as burning, is the basic chemical process of releasing energy from a fuel and air mixture. In an internal combustion engine (ICE), the ignition and combustion of the fuel occurs within the engine itself.
What causes flame in combustion?
A flame (from Latin flamma) is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction made in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma.
What is the main requirement for combustion?
We also know that three things required for combustion are fuel, oxygen, and heat. The fuel is used to burn, the air is supplied through the oxygen and the heat is used to continue the combustion process. The heat is also called ignition temperature.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.
Get App