

Boyle's Law Formula: Boyle's Law is a gas law that explains how the pressure of a gas, with a fixed mass and under constant temperature, changes concerning the volume it occupies. To put it simply, when you keep the temperature and the amount of gas steady, the pressure and volume of the gas have an inverse relationship. This fundamental concept was introduced by the Anglo-Irish chemist Robert Boyle in 1662.
The mathematical expression representing Boyle's Law Formula is:
PV=k
Where:
P symbolizes the pressure of the gas.
V symbolizes the gas's volume.
k remains unchanging as long as temperature and gas quantity remain constant.
Also Check – Periodic Acid Formula
Facts and characteristics of Boyle's Law
Inversely Proportional: Boyle's Law explains that when the gas volume decreases, its pressure increases, and when the volume increases, the pressure decreases, provided all other factors remain consistent.
Ideal Gas Assumption: Boyle's Law is strictly applicable to ideal gases. Ideal gases are hypothetical gases that follow the ideal gas law under all conditions. In reality, no gas is perfectly ideal, but many gases behave closely to the ideal gas law under typical conditions.
Constant Temperature and Amount of Gas: Boyle's Law assumes that the temperature and the amount of gas (in moles) are held constant throughout the process. Any change in temperature or the number of moles would require the use of other gas laws, such as Charles's Law or Avogadro's Law.
Pressure-Volume Curves : When Boyle's Law is graphed, it results in a hyperbolic curve. As you increase the volume, the pressure decreases, and vice versa. The product of pressure and volume (PV) remains constant at a given temperature and amount of gas.
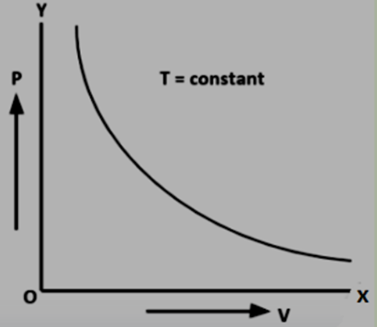
As per Boyle's law, when you change the volume of a gas while keeping the gas amount and temperature constant, it results in a proportional alteration in the gas's pressure. To put it simply, the initial pressure-volume product of a gas equals the final pressure-volume product, assuming constant temperature and moles of gas. This principle is expressed mathematically as:
P 1 V 1 = P 2 V 2
Here's what each symbol represents:
P 1 represents the gas's initial pressure.
V 1 indicates the initial volume the gas occupies.
P 2 signifies the gas's final pressure.
V 2 denotes the final volume the gas occupies.
This equation can be derived from the pressure-volume relationship proposed by Boyle's law. For a constant quantity of gas at a fixed temperature, PV=k. Therefore,
P 1 V 1 = k (initial pressure * initial volume)
P 2 V 2 = k (final pressure * final volume)
As a result,
P 1 V 1 = P 2 V 2
This equation serves as a tool to anticipate how a gas's pressure will increase against the walls of its container when the container's volume is reduced, while maintaining the same quantity of gas and temperature.
Also Check – Lithium Oxide Formula
Boyle's Law Formula Solved Examples
Example 1
A gas initially occupies a volume of 5 liters at a pressure of 800 kPa. If the gas is compressed into a new container with a volume of 2 liters, while keeping temperature and the quantity of gas constant, what will be the final pressure inside the new container?
Given:
Initial volume (V 1 ) = 5 liters
Initial pressure (P 1 ) = 800 kPa
Final volume (V 2 ) = 2 liters
By applying Boyle's Law (P 1 V 1 = P 2 V 2 ) , we can determine the final pressure, represented as P 2 :
P 2 = (P 1 * V 1 ) / V 2
P 2 = (800 kPa * 5 liters) / 2 liters = 2000 kPa
Therefore, the final pressure inside the new container is 2000 kPa.
Example 2
A gas is contained in a 10-liter tank at a pressure of 600 kPa. If the gas is transferred to a larger container with a volume of 30 liters, while maintaining a constant temperature and gas quantity, what will be the new pressure inside the larger container?
Given:
Initial volume (V 1 ) = 10 liters
Initial pressure (P 1 ) = 600 kPa
Final volume (V 2 ) = 30 liters
By applying Boyle's Law (P 1 V 1 = P 2 V 2 ) , we can determine the final pressure, represented as P 2 :
P 2 = (P 1 * V 1 ) / V 2
P 2 = (600 kPa * 10 liters) / 30 liters = 200 kPa
Therefore, the new pressure inside the larger container is 200 kPa.
Also Check – Ionic Compound Formula
Applications of Boyle's Law
Scuba Diving: When scuba divers descend underwater, the pressure increases with depth. Boyle's Law explains why a diver's air tank becomes harder to breathe from as they go deeper. The volume of the gas in the tank decreases due to increased pressure, and this requires more effort to inhale.
Inflating a Balloon: When you blow up a balloon, you are decreasing its volume by introducing more air (increasing the number of gas molecules) inside it. This increase in the number of gas molecules at a constant temperature causes an increase in pressure, making the balloon expand.
Tire Pressure: Maintaining the correct tire pressure in your car is essential for safety and fuel efficiency. As you decrease the volume of the tire (by driving or inflating), the pressure inside increases, providing the necessary support for the vehicle.
Medical Oxygen: In medical applications, oxygen is often delivered to patients at different pressures and volumes. Boyle's Law helps medical professionals adjust the settings on oxygen delivery systems to ensure patients receive the appropriate oxygen concentration.
Boyle's Law Formula FAQs
What is Boyle's Law?
What is the mathematical equation for Boyle's Law?
What are the key facts about Boyle's Law?
How can Boyle's Law be expressed mathematically for pressure and volume changes?
What are some applications of Boyle's Law?












