
Lithium Oxide Formula: Lithium oxide, also known as Lithia (Li 2 O), is an inorganic chemical compound produced through the thermal dehydration of lithium hydroxide (LiOH). This compound forms when lithium metal, a member of group 1 on the periodic table, reacts with oxygen from group 16. It possesses a molecular mass of 29.88 g/mol and exists as a white solid. When lithium metal encounters oxygen, it undergoes oxidation, leading to the formation of trace amounts of lithium peroxides. In its solid state, lithium oxide exhibits an antifluorite structure, which we'll delve into further in this article.
Also Check - Boltzmann Constant Formula
Lithium Oxide Formula and Properties
Lithium oxide, often referred to as lithia, is an inorganic compound with a molar mass of 29.88 g/mol and a solid white appearance. It has a density of 2.013 g/cm3 and serves as a coolant in nuclear reactors.
This compound can be derived from lithium peroxide or by thermally dehydrating lithium hydroxide. It is also known as Kickerite and is used in ceramics production. When it reacts with water, it exhibits violent behaviour, forming lithium hydroxide (LiOH). Lithium oxide boasts excellent thermal conductivity and is employed in cathodes for lithium-ion batteries, such as LiCoO 2 . However, prolonged exposure to lithium oxide can result in skin irritation, severe burns, and even blindness.
The chemical formula for lithium oxide is Li 2 O, composed of two lithium atoms (Li) and one oxygen atom (O). Each lithium atom donates one electron to the oxygen atom.
Also Check - Modern Periodic Table Formula
Structure of Lithium Oxide
Lithium oxide (Li 2 O) consists of two atoms, lithium (Li) and oxygen (O), with an ionic formula of 2[Li + ] [O 2− ].
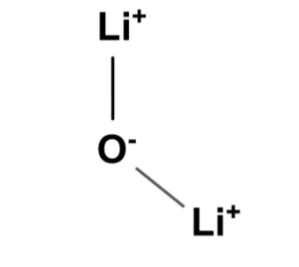
Preparation of Lithium Oxide
Lithium oxide is formed when lithium metal reacts with atmospheric oxygen, resulting in the production of lithium oxide:
4Li + O 2 → 2Li 2 O
Alternatively, it can be produced by heating lithium peroxide at temperatures ranging from 300°C to 400°C, through thermal decomposition. The purest form of lithium oxide can be obtained by thermally decomposing lithium peroxide at 450°C:
2Li 2 O 2 → 2Li 2 O + O 2
Lithium oxide can also be generated by dehydrating lithium hydroxide.
Also Check - Electronegativity Formula
Physical Properties of Lithium Oxide
Lithium oxide presents as a white crystalline solid with a molar mass of 29.88 g/mol and a density of 2.013 g/cm3. It has a high melting point of 1438°C and boiling point of 2600°C. Its crystal structure is cubic antifluorite, and it readily dissolves in water, forming lithium hydroxide.
Chemical Properties
In the gaseous phase, Li 2 O molecules are linear. Lithium oxide can react with carbon dioxide to form lithium carbonate:
Li 2 O + CO 2 → Li 2 CO 2
It also reacts with water, producing lithium hydroxide:
Li 2 O + H 2 O → 2LiOH
Side Effect of Lithium Oxide
Lithium oxide is very harsh. Breathing in its harmful fumes can irritate your breathing passages. Being around it for a long time can harm your brain and nerves. If you swallow it, it can severely damage your mouth and throat and even make holes in your oesophagus and stomach. Touching it can cause really bad burns on your skin.
Also Check - Sodium Hydroxide Formula
Uses of Lithium Oxide
Lithium oxide serves various important roles in different applications:
Thermal Barrier Coating System: In certain industries, lithium oxide is employed as a key component in thermal barrier coating systems. These systems are used to protect surfaces from extreme temperatures, providing insulation and preventing heat damage.
Coolant in Nuclear Reactors: Lithium oxide is utilized as a coolant in nuclear reactors. Its excellent heat transfer properties make it an effective choice for dissipating excess heat generated during nuclear reactions, ensuring safe and efficient reactor operation.
Thickening Agent in Grease Production: In the manufacturing of greases, lithium oxide serves as a valuable thickening agent. It enhances the viscosity of grease, giving it the necessary consistency for lubricating various mechanical components effectively.
Formation of Lithium Hydroxide: When lithium oxide reacts with water, it undergoes a chemical transformation to form lithium hydroxide. This reaction is utilised in various chemical processes and industries where lithium hydroxide is a needed component.
Flux in Ceramic Glazes: Lithium oxide finds application in the field of ceramics as a flux. A flux is a substance added to ceramics glazes to lower the melting point of certain components, enhancing the overall glazing process and resulting in desirable ceramic finishes.
Cathodes in Lithium-Ion Batteries: Lithium-containing metal oxides like LiCoO 2 are crucial components of cathodes in lithium-ion batteries. These batteries are widely used in portable electronic devices, electric vehicles, and renewable energy systems due to their high energy density and rechargeable nature.
Electrolysis for Lithium Metal Production: The electrolysis of lithium oxide is a method used to produce lithium metal, with oxygen as a by-product. This process is vital in obtaining pure lithium for various industrial applications, including the manufacturing of batteries and electronics.
Lithium oxide versatility and unique properties make it a valuable substance in several industries, ranging from energy storage to materials science, where it contributes to enhancing performance and enabling various technological advancements.
Lithium Oxide Formula FAQs
What is lithium oxide?
What are the harmful effects of lithium oxide exposure?
How is lithium oxide used in industrial applications?
What happens when lithium oxide reacts with water?
Why is lithium oxide important in battery technology?










