
In the Periodic Acid Formula, iodine is in oxidation state VII, the highest oxoacid of iodine. Iodine is found in oxidation state VII of periodic acid, which has the highest oxoacid concentration. Periodic acid is a colorless crystalline compound. Periodic acid is a periodate, meaning it can exist in two forms: orthoperiodic acid, whose chemical formula is H5IO6, and metaperiodic acid, whose chemical formula is HIO4. Water and alcohol dissolve periodic acids.
What is Periodic Acid?
Periodic acids are iodine oxides that contain more oxygen than iodic acid and differ in water content, particularly one of the crystalline compounds, HIO4 and H5IO6. Acids containing strongly oxidizing iodine, such as H5IO6 and HIO4, are considered periodic acids.Also Read - Malic Acid Formula
Periodic Acid Formula
Periodic acid has the chemical formula HIO4. Iodine is present in the periodic acid, which contains the highest amount of oxoacids of iodine. Oxoacids contain oxygen, hydrogen, and another element. There are three oxygen atoms double-bonded to the central iodine atom in the periodic acid, and one hydroxyl group single-bonded to the central atom in the periodic acid with a + 7 oxidation state.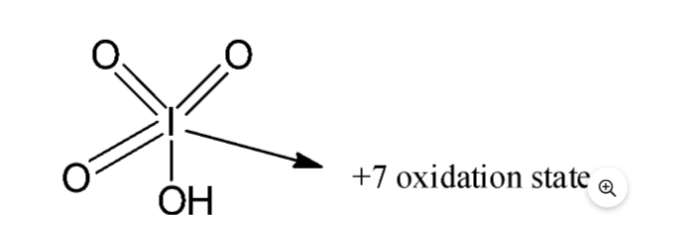
How do you write the formula of periodic acid?
- First of all, we will explain the chemical compound periodic acid. Periodic acid contains iodine, which is the highest oxoacid of iodine. Oxoacids consist of oxygen, hydrogen, and another element.
- Since the periodic acid is an oxoacid of iodine, iodine has a +7 atomic number .Having an oxidation state means that there are three oxygen atoms double bonded to the central iodine atom and one hydroxyl group single bonded to it.
- Here is the periodic acid structure :
Also Check - Elevation of Boiling Point Formula
Characteristics of Periodic Acid
Below are the few Characterstics of Periodic Acid:| Chemical formula, | HIO4 |
| Molecular weight | 191.908 g/mol |
| Chemical Names | Paraperiodic acid,Iodic(VII) acid, Hydrogen periodate |
| Solubility | Soluble in water and alcohols |
| Melting point | 128.5 °C |
| Conjugate Base | Periodate |
Applications of Periodic Acid
- It is used extensively in organic chemistry as a potent oxidizing agent. Periodic acid has diverse applications both in science and industry. In a reaction called the Malaprade reaction or oxidative glycol cleavage, it is used to cleave vicinal diols, for instance.
- The Periodic Acid-Schiff (PAS) staining technique is particularly valuable in histology and pathology for staining polysaccharides, glycoproteins, and glycolipids.
Also Check - Atomic Mass Formula
Periodic Acid: Overview
- It is a pale yellow solution of hydrogen iodide gas. Iodides are iodine compounds with oxidation state -1, such as hydrogen iodide (HI), sodium iodide (NaI), carbon tetraiodide (CI4), and nitrogen triiodide (NI3).
- Hydrogen iodate, HIO3, can be made by oxidizing I2 in an aqueous solution with chlorine. Iodine, an iodate salt of iodic acid, has three oxygen atoms bound to it.
- The prefix "meta" denotes less water, whereas"ortho" denotes more water. Heating can dehydrate orthoperiodic acid to metaperiodic acid. Periodic acid is a white crystal soluble in water and alcohol and loses water at temperatures above 100 degrees Celsius.
- Periodic acid and its salts (potassium and sodium) have powerful oxidizing properties in organic synthesis.
- As the most iodine-rich oxoacid, periodic acid can be used to analyze the structure of carbohydrates. Periodic acid has the chemical formula is HIO4, and meta-periodic acid has the formula HIO4.
- As with all periodates, orthopedic acid has the chemical formula HIO4. In 1833, Heinrich Gustav Magnus and C.F. Ammermüller discovered periodic acid.
Periodic Acid Formula FAQs
What is the chemical formula of periodic acid?
The chemical formula of periodic acid is H5IO6 for orthoperiodic acid and HIO4 for metaperiodic acid.
What are the common uses of periodic acid in chemistry?
Periodic acid is a strong oxidizing agent in various organic synthesis and laboratory reactions. It's also employed in the preparation of iodic acid and other iodine compounds.
Is periodic acid a stable compound at room temperature?
No, periodic acid is not stable at room temperature. It decomposes, especially when exposed to heat, light, or other reducing agents.
What is the structural difference between orthoperiodic acid and metaperiodic acid?
Orthoperiodic acid has five iodine atoms attached to a central oxygen atom and a tetrahedral structure. Metaperiodic acid has four iodine atoms attached to a central oxygen atom and a planar, square pyramidal structure.
Are there any safety precautions when working with periodic acid?
Yes, handling periodic acid requires caution due to its strong oxidizing properties. It should be stored away from reducing agents, and protective equipment, such as gloves and goggles, should be worn when working with it.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









