

An Electrophile Formula is a chemical species that accepts an electron pair and forms bonds with nucleophiles. Electrophiles are Lewis acids because they accept electrons. This are usually positively charged, partially positively charged, or has an atom without an octet of electrons.
What is Electrophile?
Electrophiles are electron-deficient species, which makes them attracted to electrons. They can carry either a positive or negative charge and include cations like H+ and NO+, polarisable neutral molecules like HCl, alkyl halides, acyl halides, and carbonyl compounds, reactive gases like Cl2 and Br2, oxidizing agents such as organic peracids, Lewis acids like BH3 and DIBAL as well as some species that don't meet the octet rule such as carbenes and radicals.Also Read: Discovery of Proton Formula
The movement of electrons is usually from a high density region to a low density region, making addition and substitution reactions the most common way for these species to interact with nucleophiles.Since electrophiles accept electrons, they are also known as Lewis acids.These reactions, both addition and substitution, involve electrophilic mechanisms. [caption id="attachment_17930" align="alignnone" width="300"]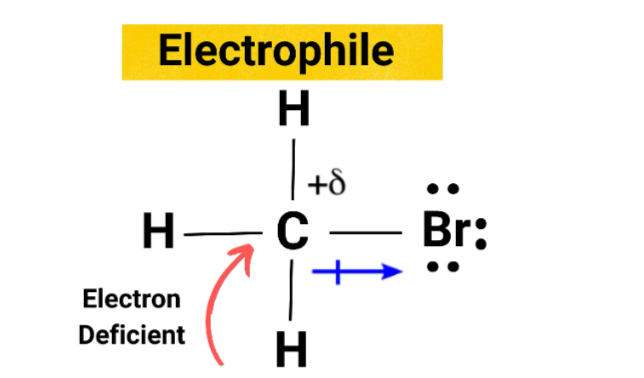 Electrophile[/caption]
Electrophile[/caption]
Types of Electrophile
Electrophiles can be classified into various types as follows: Positively Charged Electrophiles Positively charged electrophiles carries a positive charge. Positively charged electrophiles include:- H+
- SO3H+
- NO+
- NO2+
- X+
- R+
- C6H5N2+
Also Check - Sodium Hydroxide Formula
Electrophilic Reagent Examples
- Addition of Halogens
Also Check - Modern Periodic Table Formula
- Addition of Hydrogen Halides
Also Check - Electronegativity Formula
- Chiral Derivatives
Few Facts about Electrophile
- George A. Olah was the first to discover super-electrophiles, cationic electrophilic reagents with greatly enhanced reactivities in the presence of superacids.
- By proto-solvation, these compounds form as a doubly electron-deficient species.
- Mixing hydrofluoric acid and a mixture of acetic acid and boron trifluoride produces a superacid from BF3 and HF, which is capable of extracting hydride ions from isobutane through the formation of an intermediate [CH3CO2H3]2+.
- Molecules, ions, or atoms that lack electrons are electrophiles.
- Electrophiles participate in electrophilic substitutions and electrophilic additions. Since they accept electrons, they are also called Lewis acids.
- Electrophiles can have either a positive or negative charge.
- Carbon-carbon double bonds, which have a lot of electrons, are attacked by electrons.
- Molecular nucleophiles donate electron pairs to electrophiles and form chemical bonds.
E<span style=
What is an electrophile?
An electrophile is a species that seeks electrons and can accept a pair of electrons during a chemical reaction.
How do electrophiles react in organic chemistry?
Electrophiles typically engage in electrophilic reactions by forming bonds with nucleophiles, which donate electron pairs.
Can you provide examples of common electrophiles?
Common electrophiles include positively charged ions, such as H+ (proton) and carbocations, as well as molecules like acyl chlorides (RCOCl) and alkyl halides (RX, where X is a halogen).
Why are electrophiles important in organic synthesis?
Electrophiles play a crucial role in building complex organic molecules through electrophilic aromatic substitution and nucleophilic addition reactions.
Are all electrophiles highly reactive?
Electrophilic reactivity varies; some electrophiles are highly reactive and readily react with nucleophiles, while others may require specific conditions or catalysts to initiate reactions.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.











