
As an inorganic compound, sodium hydroxide is usually found at room temperature as a white solid. NaOH is the chemical formula for sodium hydroxide, which is composed of sodium Na+ cations and hydroxide anions. The chemical formula is NaOH. A variety of products are manufactured from caustic soda, also known as Iye or caustic soda, including paper, soap and detergents, pulp, explosives, liquid drain cleaners, and oven cleaners.
We will learn more about Sodium Hydroxide, its properties, preparation, reaction, and uses below. Sodium Hydroxide is one of the simplest hydroxides.Overview Of Sodium Hydroxide Formula
| IUPAC Name | Sodium Hydroxide / Sodium oxidanide |
| Chemical Formula | NaOH |
| Molar Mass | 39.997 g/mol |
| Density | 2.13 g/cm³ |
| Melting Point | 318.4 oC |
| Boiling Point | 1.388 °C |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Isotope Atom Count | 0 |
| Covalently-Bonded Unit Count | 2 |
Also Read : Malic Acid Formula
Properties of Sodium Hydroxide
Physical Properties of Sodium Hydroxide- Pure sodium hydroxide is a white crystalline solid.
- There is no odour to it.
- Solid sodium hydroxide is soluble in water, glycerol, and ethanol. Mixing it with water results in a highly exothermic reaction.
- It has a viscosity of about 78 mPas, which is higher than water.
- Some known hydrates are Heptahydrate, Pentahydrate, Tetrahydrate, Trihemihydrate, Trihydrate, Dihydrate, and Monohydrate.
- NaOH and its monohydrate can form orthorhombic crystals with space groups such as Cmcm (oS8) and Pbca (oP24).
- Carbon dioxide and water can be quickly absorbed from the air by it.
- Liquid forms are also possible.
Also Check - Discovery of Proton Formula
Chemical Properties of Sodium Hydroxide- The Na(+1) ion and OH(-1) ion form an ionic bond.
- Water and salts can be formed when it reacts with protic acids.
- With a pH of 13, it has a high acidity.
- Amphoteric hydroxides or oxides are commonly leached with sodium hydroxide.
- Hydroxide contains a covalent bond between oxygen and hydrogen.
Also Check - Electronegativity Formula
Structure of Sodium Hydroxide
Sodium hydroxide, chemical formula NaOH, is an ionic compound composed of sodium (Na+) ions and hydroxide (OH-) ions. Its structure can be represented as follows:- Sodium ion (Na+)
- Oxygen atom (O)
- Hydrogen atom (H)
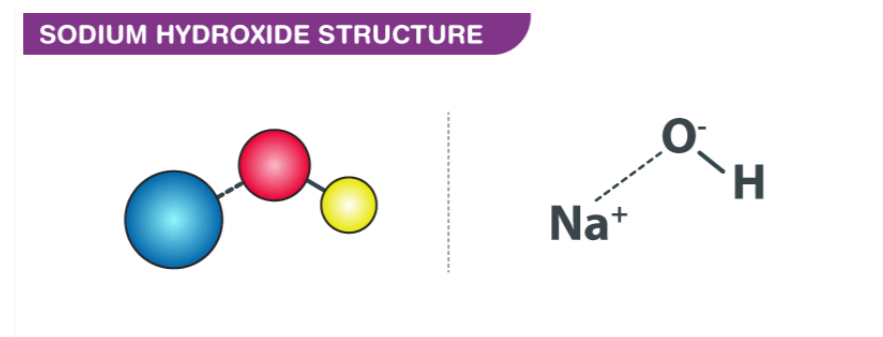 In the solid state, sodium hydroxide forms a crystalline structure where sodium ions (Na+) are surrounded by hydroxide ions (OH-) in a three-dimensional lattice. This arrangement results in a highly alkaline substance commonly known as caustic soda or lye. Sodium hydroxide is highly soluble in water, and in aqueous solution, it dissociates into sodium ions (Na+) and hydroxide ions (OH-), making it a strong base.
In the solid state, sodium hydroxide forms a crystalline structure where sodium ions (Na+) are surrounded by hydroxide ions (OH-) in a three-dimensional lattice. This arrangement results in a highly alkaline substance commonly known as caustic soda or lye. Sodium hydroxide is highly soluble in water, and in aqueous solution, it dissociates into sodium ions (Na+) and hydroxide ions (OH-), making it a strong base.
Also Check - Monatomic Gases Formula
How to Make Sodium Hydroxide?
Sodium hydroxide is most commonly manufactured through the electrolytic chloralkali process, which yields a 50% NaOH solution. During this procedure, chlorine gas is also produced. The water is then evaporated to obtain solid caustic soda, often available in the form of flakes, prills and cast blocks. Previously, the reaction between washing soda and lime was used to produce NaOH, taking advantage of its solubility compared to carbonate. In the Bayer process for extracting aluminium from bauxite ore, it serves as a purifying agent.Uses of Sodium Hydroxide
- In industry, sodium hydroxide is a popular strong base.
- NaOH is used for the manufacture of sodium salts and detergents, pH regulation, and organic synthesis.
- It is most commonly handled as a solution in bulk.
- In the petroleum industry, caustic soda is used as an additive in drilling fluids to increase alkalinity in bentonite mud systems, to increase viscosity, and to neutralize acid gas.
- It is used to make soaps and detergents.
- As well as creating artificial textile fibres (such as Rayon), it is also used in the manufacture of paper.
- The paper industry consumes 25% of the caustic soda produced by the industry, which employs 56%.
- It is used to degrease metals, refine oil, and make dyes and bleaches.
Also Check - Value of Gas Constant Formula
Storage
Especially in bulk amounts, it is important to store caustic soda carefully to avoid chemical burns. NaOH is typically stored in bottles for small-scale laboratory use, intermediate bulk containers (medium volume containers) for cargo handling and transport, or large stationary storage tanks with volumes up to 100,000 gallons for manufacturing or wastewater plants that use NaOH extensively. The most common materials used for NaOH storage include polyethylene (HDPE, usually, and XLPE, sometimes), steel, PVC (PVC), chrome steel, and fibreglass-reinforced plastic (FRP, with a resistant liner).Harmful Effects of Sodium Hydroxide
- In spite of Sodium Hydroxide's advantages, there are some limitations you should be aware of.
- One must be very careful when using sodium hydroxide because it is capable of dissolving human tissue.
- It may cause permanent blindness if it comes into contact with the eye.
- It can cause severe burns if it comes into contact with the skin.
- Solvation is an exothermic process that may lead to splashing. It may come into contact with the eyes or skin.
Sodium Hydroxide Formula FAQs
Is sodium hydroxide the same as lye?
Yes, sodium hydroxide is commonly referred to as lye. It is a strong alkaline substance used in various industrial and household applications.
What is the chemical formula of sodium hydroxide?
The chemical formula of sodium hydroxide is NaOH, representing one sodium (Na+) ion and one hydroxide (OH-) ion.
What are the common uses of sodium hydroxide?
Sodium hydroxide is used in the manufacture of soap, detergents, paper, textiles, and as a strong chemical base in various industrial processes, including water treatment.
Is sodium hydroxide safe to handle?
Sodium hydroxide is highly caustic and should be handled with care. It can cause severe burns on skin contact and should be used with appropriate safety precautions.
Can sodium hydroxide be neutralized?
Yes, sodium hydroxide can be neutralized by reacting it with an acid, such as hydrochloric acid. This reaction forms water and a salt, effectively neutralizing the strong base.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









