
Galvanic Cell: It is a device that converts chemical energy into electrical energy.
Electrolytic Cell: It is a device that converts electrical energy into Chemical energy.
Difference Between Galvanic Cell and Electrolytic Cell
| Galvanic Cell | Electrolytic Cell |
|
|
|
|
|
|
|
|
|
|
Galvanic Cell: It is a type of electrochemical cell
Construction of Galvanic Cells
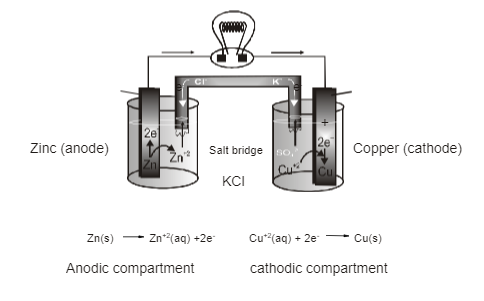
Role Of Salt Bridge:
- glass tube/polyethylene tube (U-shaped) is filled with a paste of any electrolyte (generally KCl) with agar-agar powder and dried. The electrolyte to be used in the salt bridge should be such that the speed of its cations is equal to the speed of its anions.
- Salt bridge completes the internal circuit of the cell as well as maintains electrical neutrality.
- bridge also minimizes liquid-liquid junction potential.
Cell Representation:
Electrode Potential:
Zn +2(aq) | Zn = Reduction potential of zinc electrode E Zn | Zn+2(aq) = Oxidation potential of zinc electrode For any electrode → oxidation potential = – Reduction potential E cell = R.P of cathode – R.P of anode E cell = R.P. of cathode + O.P of anode E cell = O.P of anode - O.P of CathodeStandard Electrode Potential: SRP or SOP of all other electrodes are calculated by making a cell using a standard hydrogen electrode (SHE) and the other electrode dipped In 1 Molar electrolyte and then the potential difference of this cell is measured using a potentiometer. The SRP or SOP of SHE is taken to be zero, at all temperatures but actually, it is not zero.
Electrochemical Series: The SRP values of different electrodes are calculated with reference to (SHE) and are arranged in a series (in increasing order) and the series is called electrochemical series.
| Half cell reaction | Eº RP in volts | |
| Li + +e– | ⎯⎯→ Li | –3.05 V |
| K + + e– | ⎯⎯→ K | –2.936 V |
| Ca 2+ 2e– | ⎯⎯→ Ca | – 2.87 V |
| Na + + e– | ⎯⎯→ Na | – 2.71V |
| Mg 2+ + 2e– | ⎯⎯→ Mg | – 2.36 V |
| Al 3+ + 3e– | ⎯⎯→ Al | – 1.66 V |
| 2H 2 O + 2e – ⎯⎯→ H 2 g) + 2OH – | – 0.828 V | |
| Zn 2+ + 2e– | ⎯⎯→ Zn(s) | – 0.76 V |
| Fe 2+ + 2e– | ⎯⎯→ Fe | – 0.44 V |
| PbΙ 2 (s) + 2e – ⎯⎯→ Pb(s) + 2Ι – | –0.305 V | |
| Sn 2+ + 2e ⎯⎯→ Sn(s) | – 0.14 V | |
| Pb 2+ + 2e ⎯⎯→ Pb(s) | – 0.13 V | |
| Fe 3+ + 3e– | ⎯⎯→ Fe | –0.04 V |
| 2D + + 2e– | ⎯⎯→ D 2 (g) | – 0.01 V |
| 2H + + 2e– | ⎯⎯→ H 2 (g) | 0.00 V |
| AgCl + e + | ⎯⎯→ Ag (s) + Cl – | 0.22 V |
| Cu 2+ + 2e– | ⎯⎯→ Cu(s) | 0.34 V. |
| Cu + + e– | ⎯⎯→ Cu(s) | 0.52 V. |
| Fe 3+ + e – ⎯⎯→ Fe 2+ | 0.77 V | |
| Ag + + e– | ⎯⎯→ Ag | 0.80 V |
| F 2 + 2e – | ⎯⎯→ 2F – | 2.87 V |
Features Of Electrochemical Series:
- Greater the value of S.R.P. greater will be tendency to get reduced, so, the element or compound will be stronger oxidizing agent.
- Smaller the value of SRP (higher the value of SOP) so greater will be the tendency to get oxidized hence compound or elements will be a better reducing agent.
- Anode – Electrode upper in the series
- Cathode – any lower in series in comparison to anode
- Metals which are higher up in the series can displace metals in lower in the series from their salt
- On moving down the series the electro (+ve) nature of the metals or elements decreases and also the reactivity of metals decreases.
- Metals placed above than hydrogen can release H 2 gas on reaction with dilute acid solution ex












