Titration Curve of Amino Acids : The pH of a solution during titration is represented graphically by a titration curve. When the moles of acid and base are identical and the pH is 7, the equivalence point in a strong acid-strong base titration is reached. At the equivalence point in a weak acid-strong base titration, the pH is higher than 7. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the pH of the solution as the dependent variable (because it changes depending on the composition of the two solutions). Titration is a useful tool in determining the reactivity of amino acid side chains. Since amino acids contain an ionisable group, the predominant ionic form of these molecules in solution depends on pH. Titration of amino acid shows the effect of pH on amino acid structure.
Titration Curve of Amino Acids Aim to Understand
1) To determine the titration curve for an amino acid
2) To use this curve to estimate the pKa values (pKa1, pKa2, and pKa3) of the ionizable groups of the amino acid and the amino acid’s pI.
Titration Curve
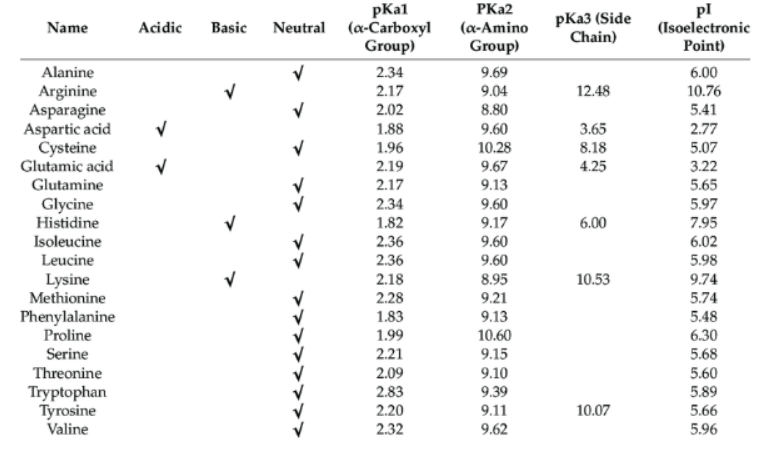
A titration curve of an amino acid is a plot of the pH of a weak acid against the degree of neutralization of the acid by a standard (strong) base. Consider the ionization of a weak organic acid, such as acetic acid, by NaOH. As more of the strong base (titrant) is added to the aqueous solution, more of the weak acid is converted to its conjugate base. During this process, a buffer system forms, and the pH of the system will follow the Henderson-Hasselbalch relationship . The titration curve of the neutralization of acetic acid by NaOH will look like this:

When a weak monoprotic acid is titrated by a base, a buffer system is formed. The pH of this system follows the Henderson-Hasselbalch equation. This curve empirically defines several characteristics (the precise number of each characteristic depends on the nature of the acid being titrated):
1) the number of ionizing groups,
2) the pKa of the ionizing group(s)
3) the buffer region(s).
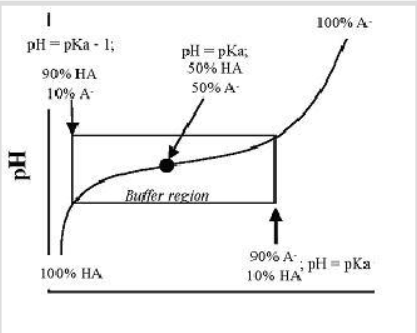
Based on the number of plateaus on a titration curve, one can determine the number of dissociable protons in a molecule. The one plateau observed when acetic acid is titrated indicates that it is a monoprotic acid (i.e., has only one dissociable H + ). Many organic acids are polyprotic (have more than one dissociable H + ). The majority of the standard amino acids are diprotic molecules since they have two dissociable protons: one on the alpha amino group and another on the alpha carboxyl group. There is no dissociable proton in the R group. This type of amino acid is called a “ simple amino acid ”. A simple amino acid is electrically neutral under physiological conditions. NOTE: Under this definition, it is possible to have a simple amino acid that is triprotic.
Which of the 20 common or standard amino acids are simple and triprotic?
The ionization of a diprotic amino acid will proceed as follows:
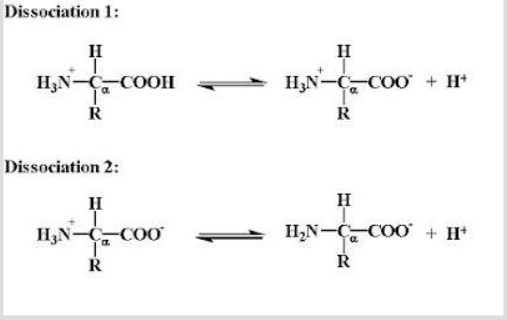
The order of proton dissociation depends on the acidity of the proton: that which is most acidic (lower pKa) will dissociate first. Consequently, the H + on the α-COOH group (pKa 1 ) will dissociate before that on the α-NH 3 group (pKa 2 ). The titration curve for this process looks similar to the following:
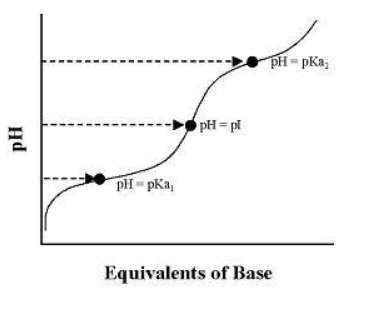
This curve reveals, in addition to the same information observed with a monoprotic acid, an additional characteristic of polyprotic acids, and that is the pH at which the net charge on the molecule is zero . This pH defines the isoelectric point ( pI ) of the molecule, a useful constant in characterizing and purifying molecules. Using a titration curve, the pI can be empirically determined as the inflection point between the pKa of the anionic and cationic forms. Mathematically, the pI can be determined by taking the average of the pKa for the anionic and cationic forms. The ionic form of the molecule having a net charge of zero is called the zwitterion .
CALCULATION OF pI:
The pH level at which a molecule's or protein's net electric charge is zero is known as the isoelectric point (pI). The molecule or protein is electrically neutral at its pI. For molecules with two ionizable groups, it is simple to compute pI using the formula pI = pKa1 + pKa2/ 2 , while for molecules with more than two ionizable groups, pI is the average of two pKa values that are close to one another .
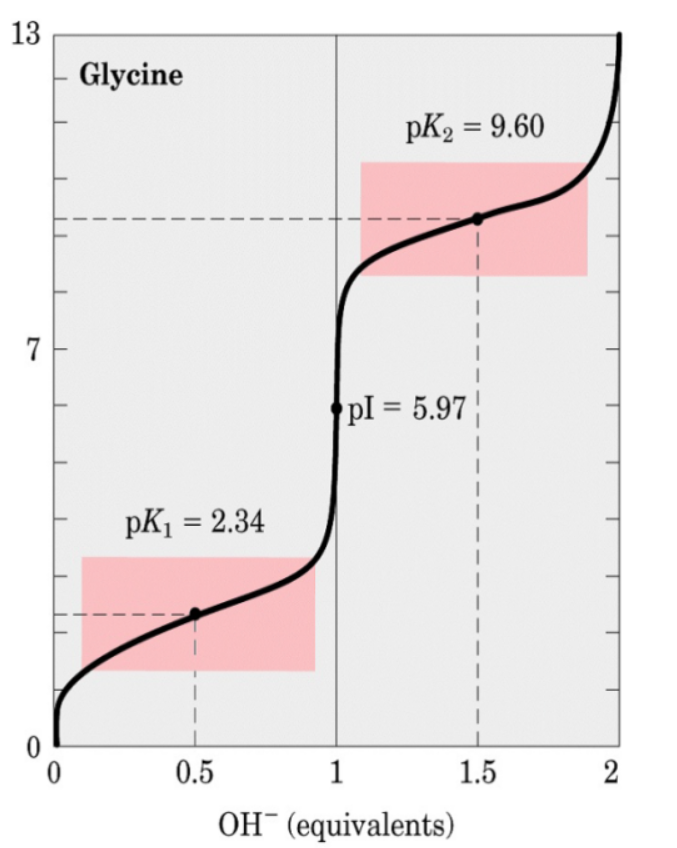
Glycine : The pKa1 of the carboxylic acid group of glycine is 2.34 and the pKa2 of the amino group is 9.60; therefore, pI (glycine) = (2.34+9.60)/2 = 5.94 .
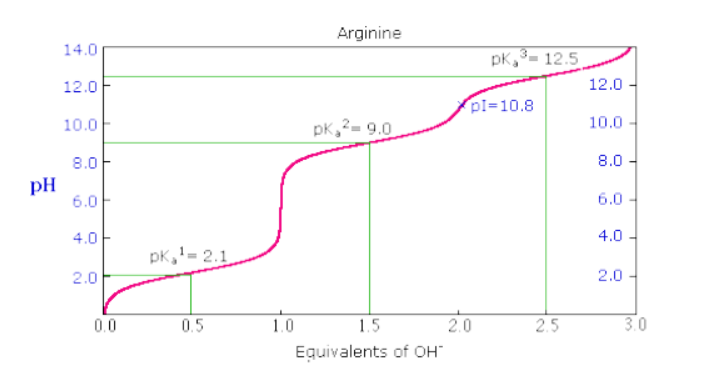
Arginine has 3 pKa values: pKa1 (carboxylic acid group) = 2.17, pKa2 (alpha-ammonium group) = 9.04, and pKa3 (guanidium R group) = 12.48. Therefore, pI (arginine) = (9.04 + 12.48)/2 = 10.76 .
A few amino acids are classified as triprotic. This is because, in addition to the ionizable protons of the α-COOH and α-NH 3 groups, they also have a dissociable proton in their R group. Although triprotic amino acids can exist as zwitterions, under physiological conditions, these amino acids will be charged. If the net charge under physiological conditions is negative , the amino acid is classified as an acidic amino acid because the R group has a proton that dissociates at a pH significantly below pH 7.
The remaining triprotic amino acids are classified as basic amino acids due to
- a) their having a net positive charge under physiological conditions; and
- b) an R group dissociable proton with a pKa near or greater than pH 7.
Titration curves for triprotic amino acids generate the same information as those for diprotic amino acids. The pI for a triprotic amino acid can be determined graphically, although this is somewhat more challenging. Graphical determination, as was the case with the diprotic acids, requires one to know the ionic forms of the amino acid and find the inflection point between the cationic and anionic forms. Mathematically, the pI for an acidic amino acid is the average of pKa 1 and pKa R (the pKa of the dissociable proton in the R group); for a basic amino acid, it is the average of pKa 2 and pKa R .
Table 1 Amino Acid Classification