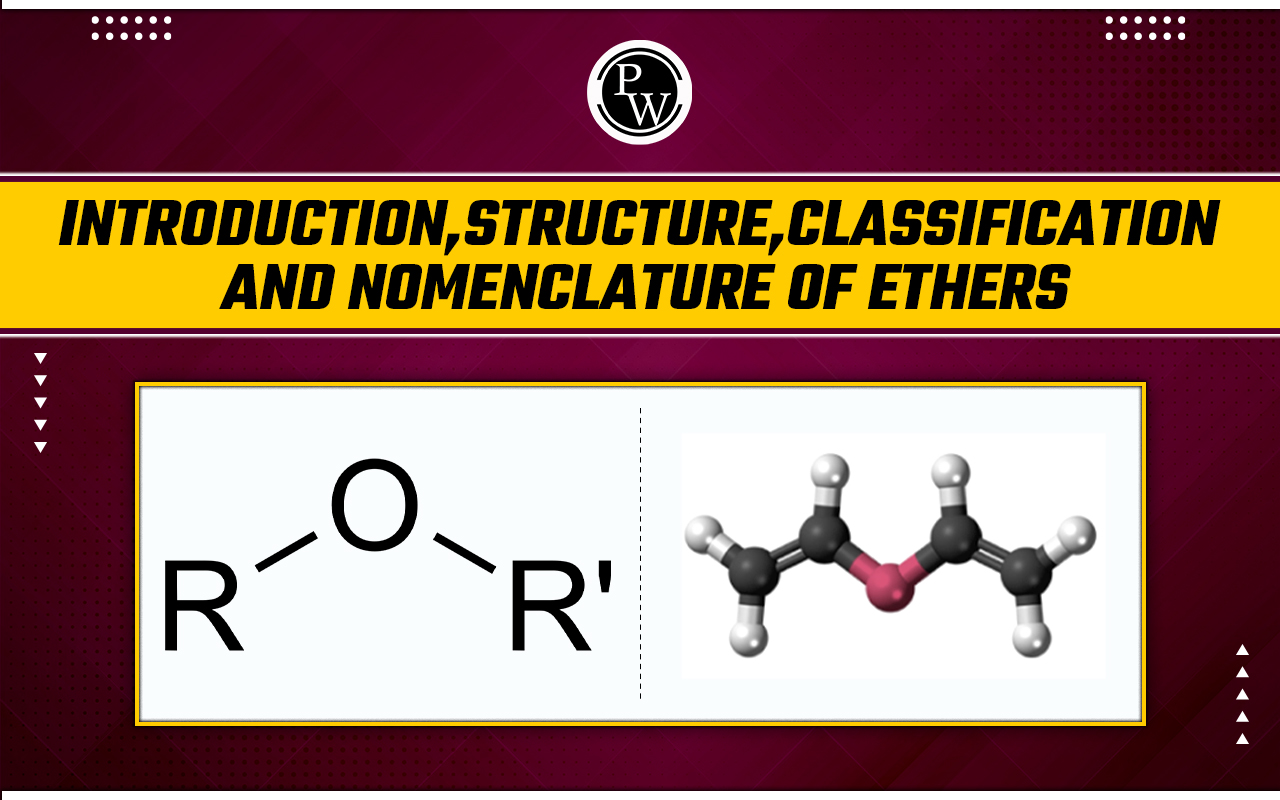
Introduction Of Ethers : Ethers are a class of compounds in organic chemistry that have an oxygen atom joined to two alkyl or aryl groups. Their general formula is R−O−R′, where the alkyl or aryl groups are denoted by R and R′. The structural properties of ethers and alcohols are comparable, as is the structure of water. An alkyl group replaces one hydrogen atom in an alcohol, while an ether replaces both hydrogen atoms with an aryl or alkyl group.
ETHER
General structure of ether 3–D model of ether
Ethers are colorless, pleasant-smelling liquids at room temperature. Ethers typically have lower boiling points, are less soluble in water, and are less dense than alcohols. Because ethers are relatively unreactive, they can be used as solvents for fats, oils, waxes, perfumes, resins, dyes, gums, and hydrocarbons. Diethyl ether is a fantastic solvent for extractions as well as a wide range of chemical reactions.
Dimethyl ether is a refrigerant and a spray propellant. In pharmacology and medicine, ethers are also crucial, particularly when used as anesthetics. For instance, ether, also referred to as ethyl ether (CH 3 CH 2 ―O CH 2 CH 3 ), was initially employed as a surgical anesthetic in 1842. Other types of ethers are also there called crown ethers, you do not need to study in detail in lower classes this is just for knowledge.
Crown ethers : Cyclic polyethers with four or more oxygen atoms spaced apart by two or three carbon atoms are known as crown ethers. Crown ethers are named using both the total number of atoms in the ring and the number of oxygen atoms. Their general formula is (OCH 2 CH 2 ) n or (OCH 2 CH 2 CH 2 ) n . An 18-membered ring with six oxygen atoms is therefore known as 18-crown-6. Every crown ether has a central cavity that can hold an alkali metal ion, like K + , and is lined with oxygen atoms.
By interacting with lone pairs of electrons on the nearby oxygen atoms, the cation is stabilized. The electrostatic potential map of the center cavity of 18-crown-6, which is displayed below, reveals the cavity's negative character. a high electron density is present.
18-Crown-6
Structure of Ethers : Ethers are a class of organic compounds with the formula R-O-R' that have an sp 3 hybridized oxygen positioned between two alkyl groups. Ethers have essentially the same geometry as alcohols and water due to the sp 3 hybridization of oxygen. In tetrahedral geometry, the R-O-R' bond angle is fairly close to what is predicted. Because of the methyl groups' steric repulsion, the bond angle of dimethyl ether is 112 0 , greater than the H-O-H bond angle in water (104.5 0 ).
Ethers have a small dipole moment due to the presence of an electronegative oxygen atom, with oxygen having the majority of the electron density.
Classification of Ethers
Classification of Ethers: Ethers are divided into two groups: symmetrical and unsymmetrical, according to the attached substituent groups. Two identical groups are joined to either side of an oxygen atom in symmetrical ethers, such as dipropyl ether, diphenyl ether, and others. Unsymmetrical ethers, such as butyl and ethyl methyl ethers, have two distinct groups bonded to either side of an oxygen atom.
Further can be classified in aliphatic, aromatic, cyclic and mixed ethers.
Aliphatic Ethers Aromatic Ethers
CH
3
CH
2
CH
2
CH
2
–O–CH
3
Cyclic ethers: Mixed ethers:
Nomenclature of ethers
Nomenclature of ethers : The IUPAC rules provide both common and systematic nomenclature for naming ethers. Ethers with simple alkyl groups are referred to by their common names. In order to accomplish this, we first identify the alkyl groups and list them alphabetically, then we add the word "ether."
|
Alkyl Group |
Name |
Alkoxy Group |
Name |
|
|
Methyl |
|
Methoxy |
|
|
Ethyl |
|
Ethoxy |
|
|
Isopropyl |
|
Isopropoxy |
|
|
Tert-Butyl |
|
Tert-Butoxy |
|
|
Pehnyl |
|
Phenoxy |
Common names : The word "ether" is followed by the alphabetical names of the alkyl groups bound to the oxygen in the common names for simple ethers.
The molecules mentioned above are instances of unsymmetrical ethers, meaning that the oxygen is bridged between two distinct alkyl groups. The prefix "di" is added if the groups are symmetrical ethers, which means they are identical. Say, for instance.
Dimethyl ether Diethyl ether Diiso propyl ether
Important common names:
Systematic IUPAC Nomenclature of Ethers
Systematic IUPAC Nomenclature of Ethers : For ethers with complex substituents, the systematic iupac nomenclature is employed. Here, one of the alkoxy (alkyl with oxygen) groups is to be treated as a substituent attached to a parent chain. As usual, the longest carbon chain is used to determine the parent chain.
Systematic IUPAC Nomenclature of Ethers Rules
- The longest carbon chain is the parent chain.
- The alkoxy groups with shorted carbon chain is a substituent.
- The substituents are places alphabetically.
It is significant to note that alkoxy groups are not ranked in the functional groups' priority chart. This implies that they are only regarded as substituents and do not alter the parent chain's suffix, in the same way as halides and alkyl groups.
E.g.
Uses of ether
1. Commercial solvents and extractants for esters, gums, hydrocarbons, alkaloids, oils, resins, dyes, plastics, lacquers, and paints are widely used ethers.
2. Dimethyl ether is a refrigerant and a spray propellant.
Ethers FAQs
Q.1: What are ethers?
Q.2: Do ethers form hydrogen bonding?
Q.3 : Do ethers have dipole moments?