
Aluminium carbonate formula Al 2 (CO 3 ) 3 is the carbonate of an aluminium salt that does not exist naturally. With high pressures of carbon dioxide and a temperature close to zero degrees Celsius, it is possible to manufacture it. To protect aluminium carbonate against external factors, creating a very complex storage system would be necessary.
Aluminium Carbonate Formula
Aluminium carbonate formula, also known as an antacid with phosphate-binding activity, is a salt that can be described in various ways. Its chemical formula, Al 2 (CO 3 ) 3 , is not well understood due to its rarity in nature. Instead, it is typically found in the form of dawsonite and hydrated basic aluminium carbonate minerals, such as scarbroite and hydroscarbroite. Preparation of aluminium carbonate involves a high-pressure furnace and a low temperature close to 0 degrees celsius.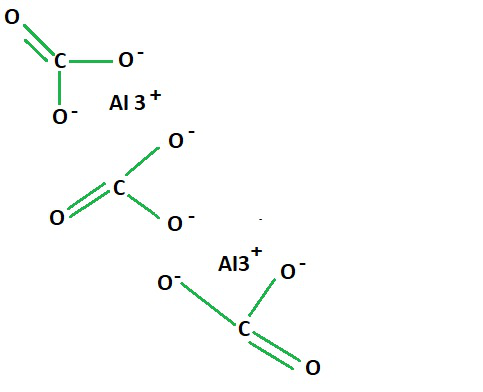
Aluminum Carbonate Formula Mass
The formula mass of aluminium carbonate, also known as aluminium carbonate, can be calculated by adding the atomic masses of all the elements in the compound. Aluminium carbonate consists of one aluminium (Al) atom, one carbon (C) atom, and three oxygen (O) atoms. The atomic masses are approximately 26.98 amu (Al), 12.01 amu (C), and 16.00 amu (O). Therefore, the formula mass is: Aluminum Carbonate Formula Mass = (1 × 26.98) + (1 × 12.01) + (3 × 16.00) amu Aluminum Carbonate Formula Mass ≈ 78.99 amuAluminium Carbonate Formula Unit
The aluminium carbonate formula is Al2(CO3)3. This means that each molecule of aluminium carbonate consists of two aluminium ions (Al³⁺) and three carbonate ions (CO₃²⁻). These ions combine in a 2:3 ratio to form the compound.Aluminum Carbonate Formula Ions
- Aluminium ion (Al³⁺): Aluminum, with an atomic number of 13, loses three electrons to achieve a stable electron configuration, forming Al³⁺ ions.
- Carbonate ion (CO₃²⁻): The carbonate ion is a polyatomic ion composed of one carbon atom (C) bonded to three oxygen atoms (O) and carries a charge of 2-. This ion is often found in compounds with metals, such as aluminium carbonate.
Aluminum Carbonate Formula Ionic
The aluminium carbonate formula is ionic because it consists of positively charged aluminum ions (Al³⁺) and negatively charged carbonate ions (CO₃²⁻) held together by ionic bonds. In ionic compounds, oppositely charged ions attract each other due to electrostatic forces, forming a stable compound.Aluminium Hydrogen Carbonate Formula
Aluminum or aluminium does not typically form a hydrogen carbonate with a 1:1 ratio like some other metals. A common aluminum compound is aluminum carbonate (Al2(CO3)3), as mentioned earlier. Hydrogen carbonates (bicarbonates) are more commonly associated with alkali and alkaline earth metals, where you have one metal ion bonded to one hydrogen carbonate ion (HCO3⁻). For example, sodium hydrogen carbonate (baking soda) has the formula NaHCO3.Aluminium Carbonate Ionic Formula
The ionic formula for aluminum carbonate is Al²³(CO₃)₃. This notation emphasizes the charge on the aluminum ion, which is +3, and the combination with the carbonate ion (CO₃²⁻). The numerical subscripts indicate the number of each ion required to balance the charges in the compound.Preparation of Aluminium Carbonate
It has not been proven that the reaction of aluminium sulfate with sodium bicarbonate can produce aluminium carbonate through double displacement reactions. Additionally, when soluble carbonates are present, they can cause a precipitation of aluminium hydroxide and release carbon dioxide. This is due to the alkaline nature of soluble carbonates.Aluminium Carbonate Properties
| Properties of Aluminium Carbonate | |
| Name | Aluminium Carbonate |
| Appearance | White Powder |
| Molecular Formula | Al 2 (CO 3 ) 3 |
| Melting Point | 58 °C |
| Boiling Point | Decomposes |
| Density | 1.5 g/cm³ |
| Molar Mass | 96.09 g/mol |
| Solubility in Water | Soluble in water |
Uses of Aluminium Carbonate
As a phosphate-binding drug, aluminium carbonate, aluminium oxide, and aluminium hydroxide are sometimes administered to dogs and cats to help them bind intestinal phosphate. Furthermore, this drug inhibits the absorption of dietary phosphate and also diminishes the absorption of phosphorus that comes from the pancreas. In addition, its use in humans is rare. This is because of concerns about its toxicity. Dogs and cats do not seem to have a toxic response to their presence. In addition, aluminum carbonate is effective in preventing urinary stones in humans. It is also useful in treating inflammations and ulcerations caused by excess stomach acid. Symptoms are treated by aluminium carbonate, but the disease itself is not affected. In addition, this drug can be taken in pill form or in liquid form. Those with kidney disease should not use aluminium carbonate and should consult a medical expert before using it. In addition, aluminium carbonate can be used to control one's body's phosphate levels.| Related Links | |
| Carbon Monoxide Formula | Chromium III Chloride Formula |
| Chloroacetic Acid Formula | Ammonia Carbonate Formula |
Aluminium Carbonate Formula FAQs
What is the name of aluminium carbonate?
The aluminium carbonate's chemical name is "Aluminum Carbonate." In some regions, it is also referred to as "Aluminium Carbonate."
What is in aluminium carbonate?
Aluminum carbonate (Al2(CO3)3) consists of two main components:
Aluminum ions (Al³⁺): These are positively charged ions formed from aluminum atoms by losing three electrons.
Carbonate ions (CO3²⁻): These are negatively charged polyatomic ions composed of one carbon atom (C) and three oxygen atoms (O), carrying a 2- charge.
What is the Valency of aluminium carbonate?
The valency of aluminum carbonate is determined by the charges of the constituent ions. Aluminum (Al) has a valency of +3 since it loses three electrons to form Al³⁺ ions. Carbonate (CO3²⁻) has a valency of -2 because it gains two electrons to achieve its 2- charge. Therefore, when they combine in a 2:3 ratio, as in Al2(CO3)3, the overall valency is balanced, resulting in a neutral compound.
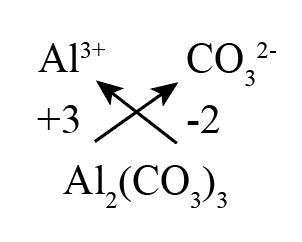
What is the formula for aluminum carbonate by the Criss Cross method?
The Criss Cross method involves swapping the valencies of the ions to determine the subscripts in the chemical formula. Using this method for aluminum carbonate, we have:
The valency of aluminum (Al³⁺) is 3.
The valency of carbonate (CO3²⁻) is 2.
So, you criss-cross these valencies to get the formula: Al2(CO3)3
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









