
It’s a colourless liquid with a pungent odour that occurs naturally. It’s an important intermediate in chemical synthesis. It is primarily found in ants and stingless bees. Due to its low toxicity, it is used as an artificial additive because of its low toxicity. Our goal in this article is to provide you with more information regarding carbonous acid, its chemical structure, its preparation, its properties, and its applications.
Carbonous Acid Formula
Carbonous acid is the strongest carboxylic acid. It has the reducing properties of aldehydes and freezes at 8.25°C, boils at 100.7°C, and has a density of 1.2126 g/cm (20°C). It is produced within the industry by mixing solid caustic soda with carbon monoxide gas (6–8 atmospheres at 120°-150°C), resulting in sodium formate (HCOONa). Vitriol decomposes sodium formate.
It is a decalcifying agent in mordant dyeing and skin preparation for tanning. It also plays a key role in producing esters, formamide, and dimethylformamide and preserving and preventing bacterial growth in livestock feed.
In place of mineral acids, it is utilized in numerous cleaning products, including limescale remover and bowl cleaner. Additionally, certain artificial flavorings and perfumes contain formate esters. For beekeepers, it serves as a miticide to combat the tracheal mite.
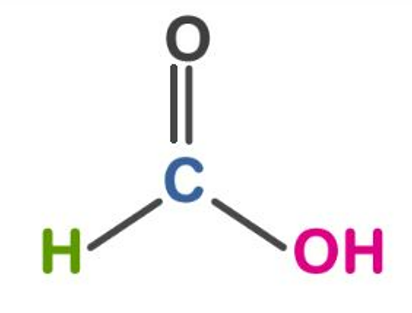
Chemical Reaction
It may be a source for a formyl group, for example within the formylation of methyl aniline to N-methylformanilide in toluene. Leuckart-Wallach and Eschweiler-Clarke reactions are chemical reactions of carbonous acids. The azeotrope, or more commonly its triethylamine azeotrope, is also used as hydrogen source during transfer hydrogenation.
CH 2 O 2 + H 2 SO 4 → H 2 SO 4 + H 2 O + CO
As a carboxylic acid, it is unique in that it can also participate in alkene reactions. With concentrated vitriol, it readily decomposes into carbon monoxide gas. The reaction between formic acids and alkenes forms formate esters. However, a variant of the Koch reaction occurs instead, where the acid adds to the alkene to supply a more considerable acid.
Also Check – Potassium Cyanide formula
Preparation of Carbonous Acid
Methanol and carbon monoxide gas are combined with a robust base to form methyl formate when methanol and carbon monoxide gas are combined.
CH 3 OH + CO → HCO 2 CH 3
Treating methyl formate with ammonia first generates formamide, which is then hydrolyzed with sulfuric acid.
HCO 2 CH 3 + NH 3 → HCONH 2 + CH 3 OH
2HC(O)NH 2 + 2H 2 O + H 2 SO 4 → 2HCO 2 H + (NH 4 ) 2 SO4
After the reaction at heat, we get propenyl alcohol. The reaction is as follows:
C 2 O 4 H 2 → CO 2 H 2 + CO 2
Also Check – Malic Acid formula
Carbonous Acid Properties
|
Properties of Carbonous Acid |
|
| Name | Carbonous Acid |
| Other Names | Formic Acid, Hydrogen carboxylic acid |
| Appearance | Colourless fuming liquid |
| Chemical Formula | CH 2 O 2 |
| Melting Point | 8.4 °C |
| Boiling Point | 100.8 °C |
| Density | 1.220 g/mL |
| Molar Mass | 46.025 g.mol–1 |
| Solubility in Water | Miscible |
Also Check – Potassium Cyanide formula
Carbonous Acid Uses
Some uses of the Carbonous Acid Formula are:
- In livestock feed, it acts as an antibacterial and preservative
- It is used in the production of rubber as a coagulant
- Leather is made from this material and can effectively treat warts.
Carbonous Acid Formula FAQs
Q1. What is the chemical formula of carbonous acid?
Q2. Is carbonous acid a stable compound at room temperature?
Q3. How is carbonous acid different from carbonic acid?
Q4. What are the main products of the decomposition of carbonous acid?
Q5. Is carbonous acid commonly encountered in everyday life?










