
Important Questions for Class 11 Chemistry Chapter 8: Chapter 8 of Class 11 Chemistry, "Organic Chemistry: Some Basic Principles and Techniques," introduces the fundamentals of organic chemistry, focusing on the structure, classification, and nomenclature of organic compounds. Key topics include functional groups, types of isomerism (structural and stereoisomerism), and the concept of homologous series.
It also covers the methods of preparation, identification, and analysis of organic compounds, such as distillation, crystallization, and chromatography. The chapter emphasizes the importance of functional groups in determining the properties and reactivity of organic molecules. This foundation is crucial for understanding more advanced topics in organic chemistry.Important Questions for Class 11 Chemistry Chapter 8 Overview
Important Questions for Class 11 Chemistry Chapter 8 PDF
Below, we have provided a PDF of important questions for Class 11 Chemistry Chapter 8, "Organic Chemistry: Some Basic Principles and Techniques." These questions cover key concepts, such as types of organic compounds, functional groups, nomenclature, and basic reactions, which are essential for understanding the fundamentals of organic chemistry. Download the PDF for detailed preparation.Important Questions for Class 11 Chemistry Chapter 8 PDF
Important Questions for Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles and Techniques
Below is the Important Questions for Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles and Techniques -
3. Write the bond line formula for
Ans.
4. How are organic compounds classified?
Ans: (i) Acyclic or open chain compounds
(ii) Alicyclic or closed chain or ring compounds.
(iii) Aromatic compounds.
5.Define homologous series?
Ans: A group or a series of organic compounds each containing a characteristic functional group forms a homologous series and the members of the series are called homologous.
6. Write an example of a non-benzenoid compound.
Ans:
Structure of Tropolene
7. What is the cause of geometrical isomerism in alkenes?
Ans: Alkene has a π − bond and the restricted rotation around the π− bond gives rise to geometrical isomerism.
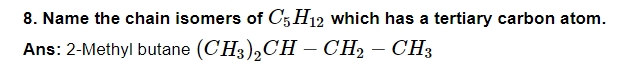
10. Define carbocation.
Ans: A species having a carbon atom possessing a sextet of electrons and a positive charge is called carbocation.
11. What are the nucleophiles?
Ans: The electron rich species are called nucleophiles. A nucleophile has affection for a positively charged centre.
Example- O H − , I − , C N −
12. How can the mixture of kerosene oil and water be separated?
Ans: The mixture of kerosene oil and water can be separated by using a separating funnel.
13. Lassaigne’s test is not shown by diazonium salts. Why?
Ans: Diazonium salts usually leave N 2 on heating much before they have a chance to react with the fused sodium metal. Therefore, diazonium salts do not show positive Lassaigne’s test for nitrogen.
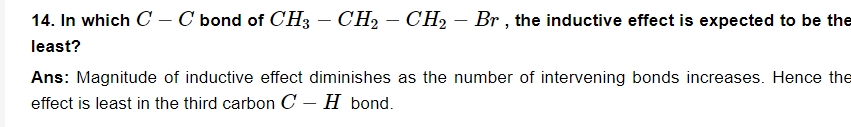
15. Can you use potassium in place of sodium for fusing an organic compound in Lassaigne’s test?
Ans: No, because potassium is more reactive than sodium.
16. Give the reason for the fusion of an organic compound with sodium metal for testing nitrogen, sulphur and halogens.
Ans: The elements present in the compound are converted from covalent form into ionic form by fusing the compound with sodium metal.
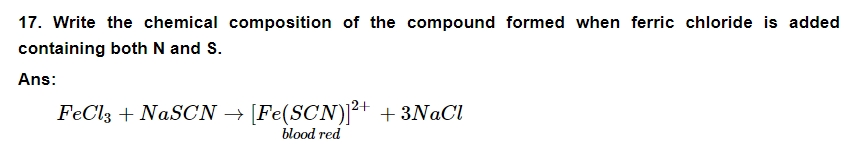
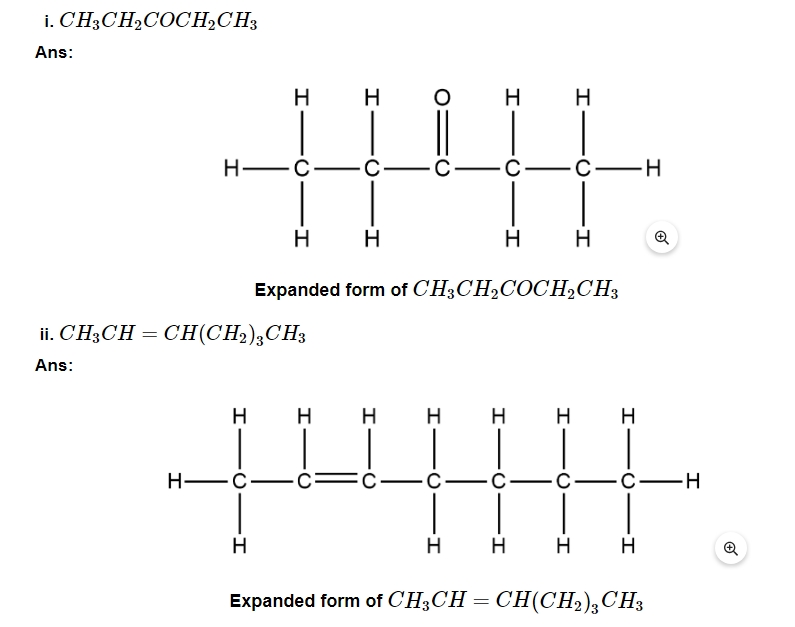
18. Write the expanded form of the following condensed formulas into their complete structural formulas.
2. How does hybridization affect electronegativity?
Ans: The greater the s-character of the hybrid orbitals, the greater is the electronegativity. Thus, a carbon atom having a sp-hybrid orbital with 50% s -character is more electronegative than that possessing s p 2 s or sp3 hybridized orbitals.
3. Why is sp hybrid orbital more electronegative than sp2 or s p 3 hybridized orbitals?
Ans: The greater the s-character of the hybrid orbitals, the greater is the electronegativity. Thus, a carbon atom having a sp-hybrid orbital with 50% s -character is more electronegative than that possessing sp2 or sp3 hybridized orbitals.
Example: Hydroxyl group (-OH), Aldehyde group (-CHO), Carboxylic acid group (-COOH), etc.
4. Give two examples of aliphatic compounds.
5. Write an example of an alicyclic compound.
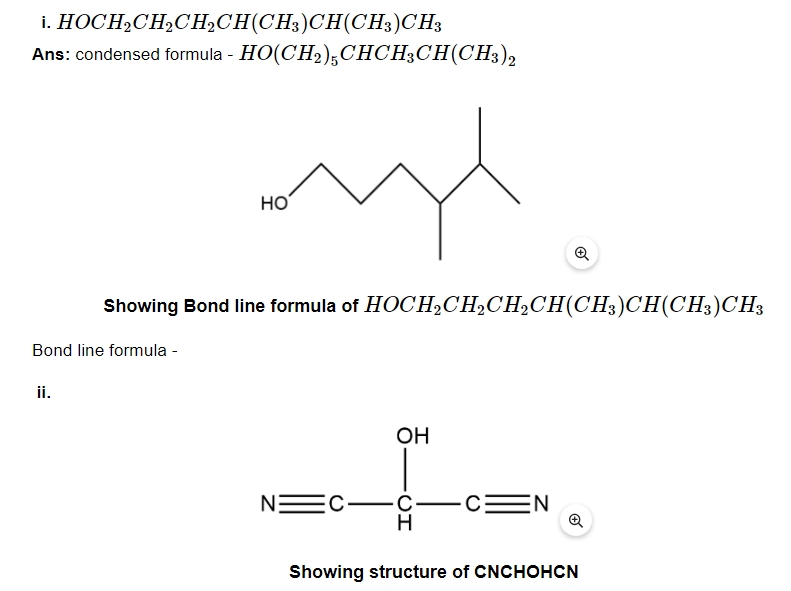
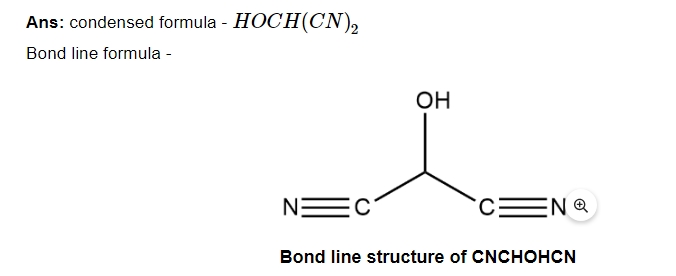
6. For each of the following compounds write a condensed formula and also their bond line formula.
Benefits of Solving Important Questions for Class 11 Chemistry Chapter 8
Solving important questions for Class 11 Chemistry Chapter 8, "Organic Chemistry: Some Basic Principles and Techniques," offers several benefits to students. Here are the key advantages: 1. Strengthens Conceptual Understanding The chapter covers fundamental concepts such as functional groups, types of organic reactions, and basic techniques used in organic chemistry. Solving important questions helps reinforce these concepts, allowing students to grasp the theoretical aspects more clearly. 2. Improves Problem-Solving Skills Organic chemistry often involves logical reasoning and applying principles to solve problems. Regular practice of important questions enables students to improve their analytical and problem-solving skills, which are crucial for mastering the subject. 3. Boosts Exam Preparation By focusing on important questions, students can familiarize themselves with the types of problems likely to appear in exams. This targeted preparation boosts confidence and helps in time management during exams. 4. Builds a Strong Foundation for Advanced Topics Organic chemistry forms the basis for more complex topics in higher studies, such as reaction mechanisms and spectroscopy. Solving key questions ensures that students have a solid foundation, making it easier to understand advanced concepts later. 5. Enhances Accuracy and Speed With practice, students develop better accuracy in solving problems and improve their speed, which is essential for performing well in competitive exams.Important Questions for Class 11 Chemistry Chapter 8 FAQs
What is the basic concept of organic chemistry?
What is the basis of organic chemistry?
What are the basic principles of organic chemistry?
What is the major focus of organic chemistry?









