
Important Questions for Class 11 Chemistry Chapter 6: Chapter 6 of Class 11 Chemistry, titled Equilibrium, covers the concept of chemical equilibrium, where the rates of the forward and reverse reactions are equal. Key topics include the Law of Chemical Equilibrium, Le Chatelier's Principle, and the equilibrium constant (K).
Students should focus on understanding the factors that affect equilibrium, such as concentration, temperature, and pressure. Important questions often ask about the calculation of equilibrium constants, the effect of changes in conditions, and the principles of dynamic equilibrium in both reversible reactions and chemical systems. Practical applications like the Haber process and equilibrium in acids and bases are also essential.Important Questions for Class 11 Chemistry Chapter 6 Overview
Important Questions for Class 11 Chemistry Chapter 6 Equilibrium, is a crucial topic that lays the foundation for understanding chemical reactions and their behavior under various conditions. Important questions typically focus on concepts like the law of equilibrium, Le Chatelier’s Principle, and equilibrium constants for reversible reactions. Understanding these principles is key for solving problems related to reaction rates, concentration changes, and the effects of temperature, pressure, and concentration on chemical equilibria. Mastery of this chapter is essential for scoring well in exams and for progressing to more advanced topics in physical chemistry, especially in competitive exams.Important Questions for Class 11 Chemistry Chapter 6 PDF
In this article, we have provided a comprehensive set of important questions for Class 11 Chemistry Chapter 6 Equilibrium. These questions cover key topics and concepts that will help you strengthen your understanding of equilibrium principles and their applications. Below, you will find a PDF containing these questions for your convenience and easy reference to aid in effective preparation.Important Questions for Class 11 Chemistry Chapter 6 PDF
Important Questions for Class 11 Chemistry Chapter 6 With Answers
Below is the Important Questions for Class 11 Chemistry Chapter 6 Equilibrium -- Define dynamic equilibrium.
Ans: Dynamic equilibrium is that stage of the solution when the reactants are present in a closed vessel at any particular temperature reacts with each other to give rise the products and in this process the concentrations of the reactants goes on decreasing while of products it keep on increasing and after some time a stage occurs where we don’t find any change in the concentration of reactants or products. This stage is called the dynamic equilibrium.
- What is physical equilibrium? Give an example.
Ans: The equilibrium which occurs between two different physical states of same substances. Example of physical equilibrium can be shown as below:
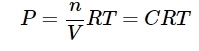
- What is meant by the statement ‘Equilibrium is dynamic in nature’?
Ans: That stage of the reaction at which reaction will not stop rather than stopping it will be in a continuous manner then equilibrium is said to be dynamic in nature. In this case reaction appears to stop because the rate of forward reaction is equal to the rate of backward reaction.
- How does dilution with water affect the pH of a buffer solution?
Ans: Rate of dilution shows no effect on the pH of buffer solution as pH depends upon the ratio of salt, acid or salt, base and dilution will not affect this ratio.
- On what factor does the boiling point of the liquid depends?
Ans: The boiling point of liquid generally depends upon the atmospheric pressure of the given liquid.
- State Henry’s law.
Ans: Henry’s law states that the mass of a gas dissolved at any temperature in a given mass of any solvent is directly proportional to the gas present in the solvent.
- What happens to the boiling point of water at high altitude?
Ans: Boiling point generally depends upon the depth of the substance and at high altitude boiling point of water goes on decreasing.
- On which factor does the concentration of solute in a saturated solution depends?
Ans: Concentration of solute in a saturated solution generally depends upon the temperature of that solution. If the temperature is high then the solute dissolves easily.
- What conclusion is drawn from the following –
Solid⇌LiquidSolid⇌Liquid
Ans: This states that melting point is fixed at any constant pressure as there is proper melting point for solid at given pressure like ice melts at 0 degree Celsius.
- State the law of chemical equilibrium.
Ans: At any temperature product of the concentrations of the reaction raised to the power of their respective stoichiometric coefficient in the balanced chemical equation which is further divided by the product of concentrations of the reactants which are also raised to their individual stoichiometric coefficients has a constant value. This is known as the equilibrium law or law of chemical equilibrium.


15. Define reaction quotient.
Ans: Reaction quotient generally represented by the letter Q is used to measure the relative amounts of products and reactants present in a reaction at any given time.
16. If Q c > K c Qc>Kc , what would be the type of reaction?
Ans: This resembles reaction in the side of reactants which tells that reaction is of reverse type i.e., on the left hand side or we can say it as backward reaction.
17. What inference you get when Q c > K c Qc>Kc ?
Ans: This relation suggests that reaction is at equilibrium.
18. State Le chatelier’s principle.
Ans: Le chatelier’s principle is generally to predict the changes occurring in chemical equilibrium of any reaction.
19. Can a catalyst change the position of equilibrium in a reaction?
Ans: Catalyst cannot change the position of equilibrium it just affects the rate of reaction.
Benefits of Solving Important Questions for Class 11 Chemistry Chapter 6
Solving important questions for Class 11 Chemistry Chapter 6 (Equilibrium) offers several benefits, especially when preparing for exams like CBSE or competitive exams. Here are some key benefits:Concept Reinforcement : Solving important questions helps reinforce fundamental concepts of equilibrium, including Le Chatelier's Principle, the equilibrium constant (K), and the conditions for equilibrium. Repeated practice ensures a deeper understanding of these concepts.
Application of Theories : By practicing questions, you can apply theoretical knowledge to real-life scenarios or laboratory-based problems, improving problem-solving skills and making it easier to understand complex concepts like the equilibrium constant and factors affecting equilibrium.
Time Management : Regular practice of important questions helps in improving time management skills. Knowing how much time to allocate to each type of problem during an exam helps in completing the paper efficiently.
Improves Analytical Skills : Questions on equilibrium often require critical thinking and a methodical approach. Solving such questions develops your analytical abilities and helps in identifying patterns or strategies to solve different types of problems.
Boosts Confidence : When you consistently solve important questions and check your answers, you build confidence in your ability to tackle questions on the exam. Confidence plays a crucial role in improving performance.
Important Questions for Class 11 Chemistry Chapter 6 FAQs
What are the factors affecting equilibrium?
What will affect equilibrium?
What are the 4 requirements for an equilibrium system?
What does equilibrium depend on?









