
Important Questions for Class 11 Chemistry Chapter 9: Chapter 9 of Class 11 Chemistry, titled "Hydrocarbons," focuses on the study of organic compounds consisting only of carbon and hydrogen. It covers the classification of hydrocarbons into alkanes, alkenes, alkynes, and aromatic hydrocarbons.
Key topics include the methods of preparation, properties, and reactions of each class of hydrocarbons, such as combustion, addition, and substitution reactions. The chapter also introduces the concept of isomerism, emphasizing structural and geometric isomerism. Understanding hydrocarbons is essential for grasping more advanced organic chemistry concepts and their applications in industries like petrochemicals and fuel production.Important Questions for Class 11 Chemistry Chapter 9 Overview
Chapter 9 of Class 11 Chemistry, Hydrocarbons, is crucial as it lays the foundation for understanding organic chemistry. Important topics include the classification of hydrocarbons, nomenclature, isomerism, and reactions of alkanes, alkenes, and alkynes. Mastery of these concepts is essential for solving problems related to molecular structure, reactivity, and stability. The chapter also helps in preparing for entrance exams, where questions on organic compounds and their reactions are common. Understanding these topics not only strengthens theoretical knowledge but also enhances practical problem-solving skills in organic chemistry.Important Questions for Class 11 Chemistry Chapter 9 PDF
In this section, we have compiled important questions from Chapter 9 Hydrocarbons for Class 11 Chemistry. These questions cover various key topics and concepts, helping students enhance their understanding and prepare effectively for exams. To assist in your studies, we have also provided a PDF with additional practice questions and detailed explanations. Download the PDF below for a comprehensive set of questions to boost your preparation.Important Questions for Class 11 Chemistry Chapter 9 PDF
Important Questions for Class 11 Chemistry Chapter 9 Hydrocarbons
Below is the Important Questions for Class 11 Chemistry Chapter 9 Hydrocarbons - 1. Classify the hydrocarbons according to the carbon – carbon bondAns: According to the carbon–carbon bond that occurs between them, hydrocarbons are divided into three categories:
(a) saturated hydrocarbon
(b) unsaturated hydrocarbon
(c) aromatic hydrocarbon
2. What are cycloalkanes?
Ans: Cycloalkanes are formed when carbon atoms form a closed chain or ring.
3. Why does carbon have a larger tendency of catenation than silicon although they have the same number of electrons?
Ans: It's because the C-C bond is smaller but stronger ( 335 KJ mol-1335 KJ mol-1 ) than the Si bond ( 225.7 KJ mol-1225.7 KJ mol-1 ).
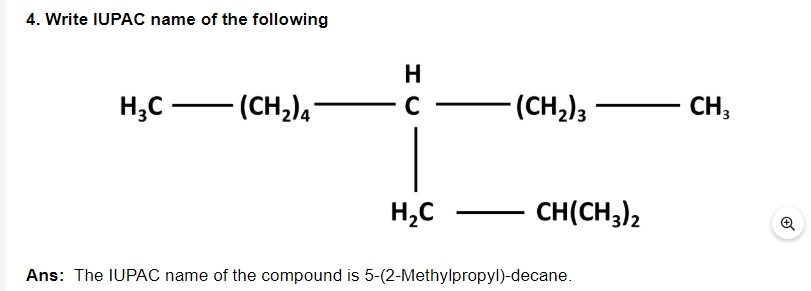
5. What is hydrogenation?
Ans: To produce alkanes, dihydrogen gas is added to alkenes and alkenes in the presence of finely split catalysts such as Pt, Pd, or Ni. This is referred to as hydrogenation.
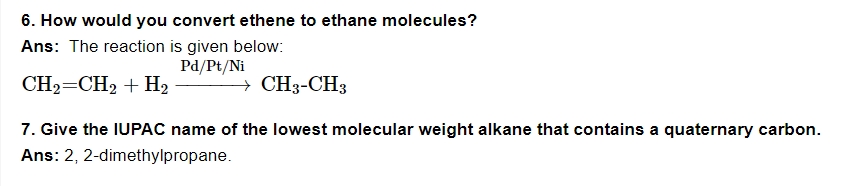
8. Methane does not react with chlorine in the dark. Why?
Ans: Chlorination of methane is a substitution process involving free radicals. Chlorine cannot be transformed into free radicals in the dark, therefore the reaction does not take place.
9. Which conformation of ethane is more stable?
Ans: Staggered conformation.
10. State Le chatelier’s principle.
Ans: It asserts that a change in any of the elements that define a system's equilibrium conditions will cause the system to alter in such a way that the effect of the change is reduced or counteracted.
11. Can a catalyst change the position of equilibrium in a reaction?
Ans: No, a catalyst cannot alter the equilibrium position of a chemical process. A catalyst, on the other hand, has an effect on the reaction rate.

14. Can a catalyst change the position of equilibrium in a reaction?
Ans: No, a catalyst cannot alter the equilibrium position of a chemical process. A catalyst, on the other hand, has an effect on the reaction rate.

18. Arrange the following halogen atom to determine rate of the reaction. Iodine, Chlorine, Bromine.
Ans: The order will be Iodine > Bromine > Chlorine
19. What is β -elimination reaction?
Ans: When the atom of hydrogen is removed from β -carbon (carbon atom next to the carbon to which halogen is attached).
20. What is the number of σ and π bond in N≡C-CH-C≡N ?
Ans: The numbers of σ bonds are 7 and the numbers of π bonds are 5.
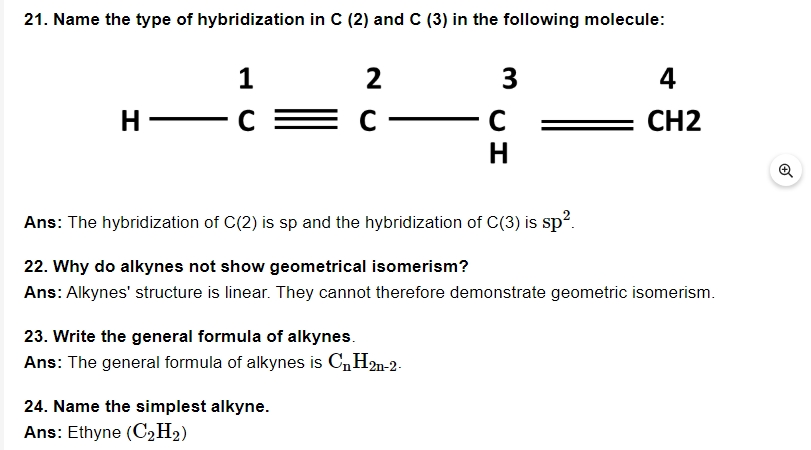
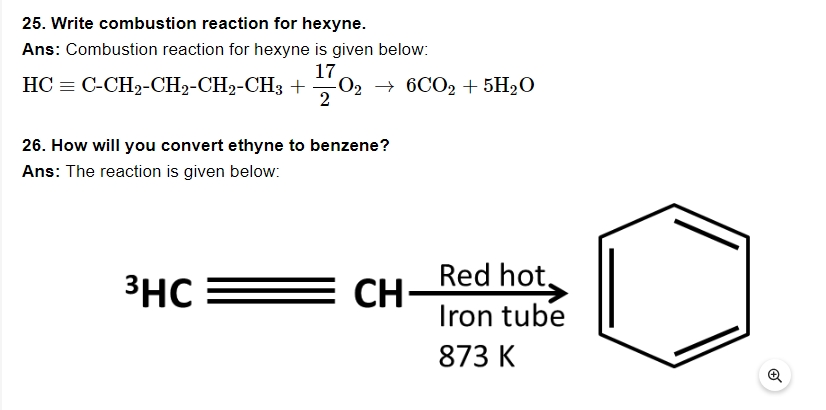
27. What are benzenoids?
Ans: Benzene ring is recognised as an aromatic hydrocarbon compound.
28. Although benzene is highly unsaturated; it does not undergo addition reactions. Give a reason.
Ans: Contrary to olefins, benzene π -electrons are relocated (resonance), and hence they are not reactive in terms of further reactions.
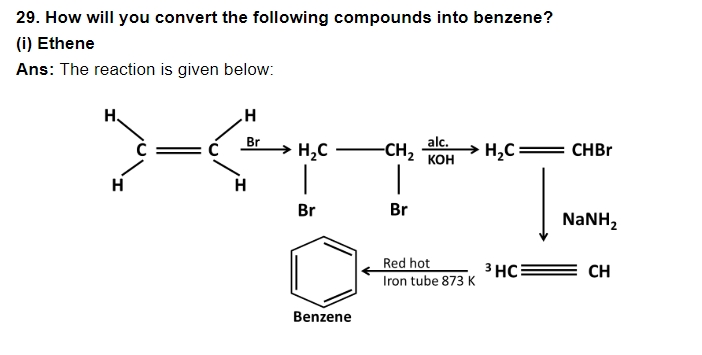
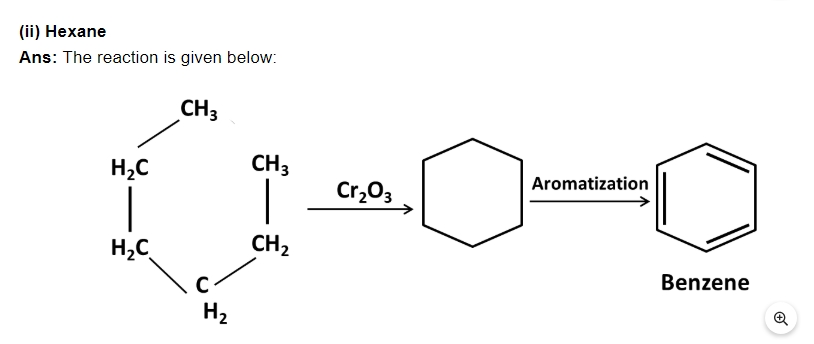
30. N – pentane has higher boiling point than neopentane but the melting point of neopentane is higher than that of n – pentane.
Ans: The surface area and van der waal forces of attraction in neopentane are much less than in n-pentane due to the existence of branches. As a result, neopentane has a lower boiling point than n-pentane.
The packing of molecules in the crystal lattice determines M.P. Because neopentane is more symmetrical than n-pentane, it packs significantly closer in the crystal lattice than n-pentane, giving it a substantially higher m.p. than n-pentane.
31. Explain the term polymerization with two examples.
Ans: Polymerization is the process of combining two or more molecules of unsaturated compounds to produce a larger complex given the right conditions. The resulting product is known as a polymer, and the process is known as polymerization.
(a) Additional polymerization: Nothing is wasted during the process since the larger molecule (polymer) is an exact multiple of the smaller molecule.
(b) Condensation polymerization: Molecules such as water, hydrochloric acid, and others are typically lost during this process. The polymer is not an exact multiple of the smaller molecule during polymerization.
Benefits of Solving Important Questions for Class 11 Chemistry Chapter 9
Here are the benefits of solving important questions for Class 11 Chemistry Chapter 9 Hydrocarbons:Concept Reinforcement : Solving questions helps in reinforcing key concepts such as the classification of hydrocarbons, functional groups, and reactions specific to alkanes, alkenes, and alkynes.
Improves Problem-Solving Skills : Regular practice enhances problem-solving abilities, especially in reaction mechanisms and identifying the type of hydrocarbons in different scenarios.
Boosts Exam Preparation : Important questions are often representative of the types of problems encountered in exams, making them essential for focused revision and time management.
Clarifies Reaction Mechanisms : Questions related to reactions like substitution, addition, and elimination help in better understanding the mechanisms involved in the chemical transformations of hydrocarbons.
Helps in Identifying Key Topics : By solving a variety of important questions, students can identify frequently tested topics, ensuring they prioritize their study time effectively.
Increases Speed and Accuracy : Regular practice allows students to improve their speed and accuracy in solving questions, an essential skill for time-bound exams.
Important Questions for Class 11 Chemistry Chapter 9 FAQs
What type of hydrocarbon is used as a lubricant Class 11?
Is hydrocarbon a difficult chapter?
What is the most important source of hydrocarbon?
Which of the hydrocarbon is mainly used as fuel?









